CONFERENCE UPDATE (ASCO 2025)
Savolitinib + osimertinib prolongs PFS in METamp, EGFR-mutated NSCLC post-first-line EGFR TKI failure: Results from the phase 3 SACHI trial
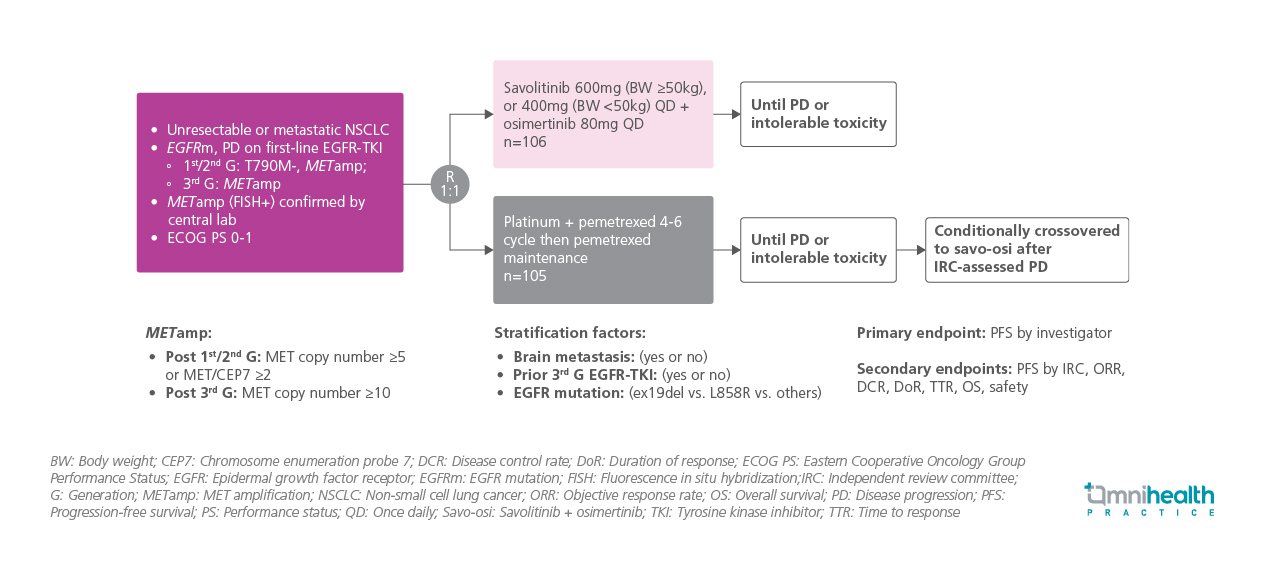
STUDY DESIGN
Mesenchymal-epithelial transition factor gene amplification (METamp) has emerged as a key resistance mechanism in up to 22% of pretreated patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC).1 This alteration is associated with early disease progression and poor prognosis.1 Despite this, biomarker-guided treatment options following progression on EGFR tyrosine kinase inhibitors (TKIs) remain limited.1 To date, there has been no randomized clinical trial specifically addressing METamp in this setting.1
Savolitinib, a selective MET inhibitor, and osimertinib, a third-generation (3G) EGFR-TKI, have previously demonstrated promising clinical activity against EGFR-mutated NSCLC with METamp in the TKI-refractory setting.1 The SACHI study is a phase 3, multicenter, randomized, open-label trial conducted in China to evaluate the efficacy and safety of savolitinib + osimertinib vs. platinum-based doublet chemotherapy in patients with locally advanced or metastatic EGFR-mutant NSCLC and METamp following first-line EGFR-TKI therapy failure.1
Eligible patients had unresectable or metastatic NSCLC with documented EGFR mutation and disease progression (PD) on first-line EGFR-TKI therapy.1 For those previously treated with first- or second-generation EGFR-TKIs, inclusion required being T790M-negative with METamp, whereas patients progressing on 3G EGFR-TKIs were required to have METamp alone.1 METamp was confirmed centrally by fluorescence in situ hybridization (FISH+).1 The METamp threshold was defined as MET gene copy number ≥5 or MET/CEP7 ratio ≥2 following first-/second-generation TKI or MET copy number ≥10 after 3G TKI exposure.1 All patients were required to have an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0-1.1
A total of 211 patients were randomized 1:1 to receive either oral savolitinib (600mg daily for ≥50kg or 400mg for <50kg) + osimertinib (80mg daily), or platinum + pemetrexed for 4-6 cycles followed by pemetrexed maintenance.1 Treatment continued until PD or unacceptable toxicity.1 Randomization was stratified by presence of brain metastases, prior 3G EGFR-TKI use and EGFR mutation subtype (exon 19 deletion, L858R, or other).1 Patients in the chemotherapy arm were allowed to cross over to receive savolitinib + osimertinib upon PD confirmed by an independent review committee (IRC).1 Baseline demographics and clinical characteristics were balanced across treatment arms.1
The primary endpoint was progression-free survival (PFS) as assessed by investigators.1 The secondary endpoints presented were PFS by IRC, objective response rate (ORR), duration of response (DoR), disease control rate (DCR), overall survival (OS), and safety.1
FINDINGS
| Primary endpoint : |
|
|
|
|
| Secondary endpoints : |
|
|
|
|
|
|
| Safety : |
|
|
|
|

