CONFERENCE UPDATES: ASCO 2023
Confirmed OS benefits of adjuvant osimertinib in resected EGFR-mutated stage IB-IIIA NSCLC patients: The ADAURA trial
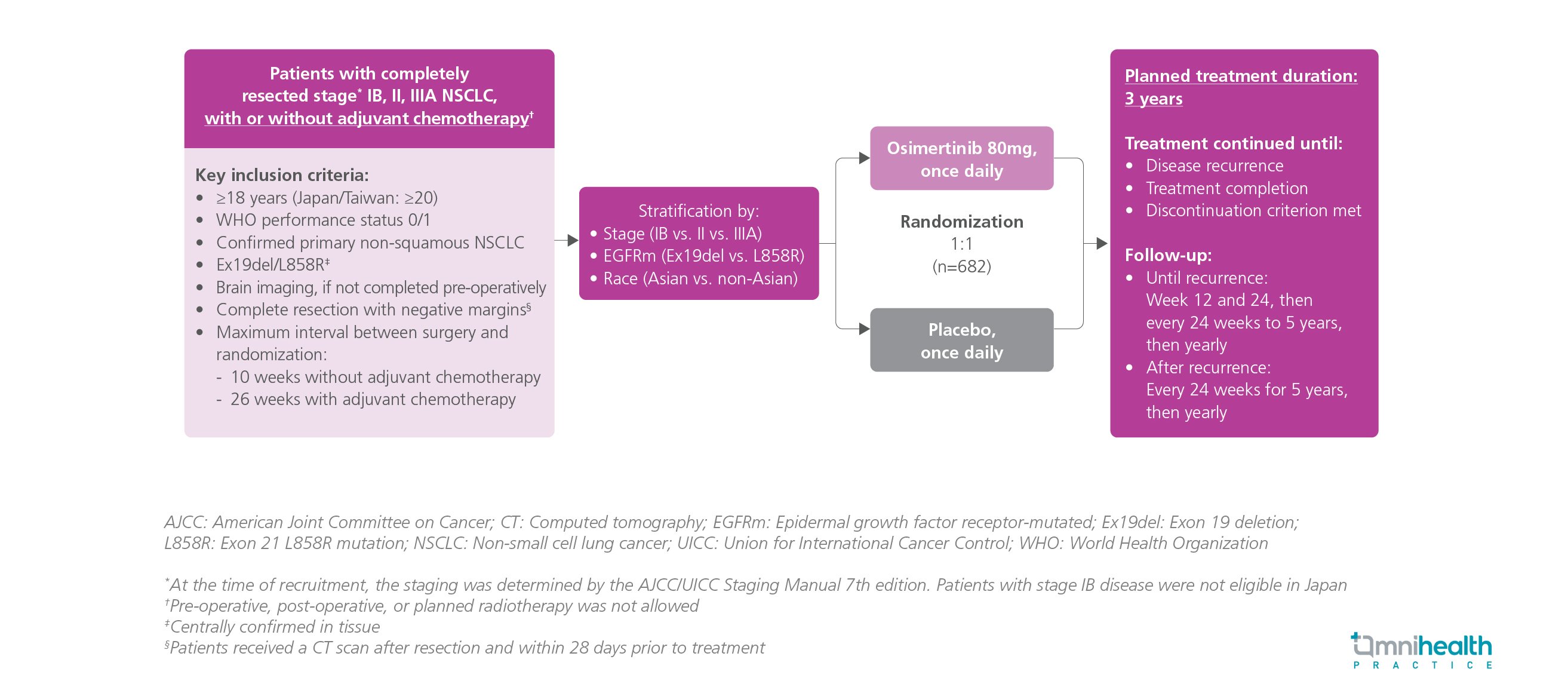
STUDY DESIGN
The ADAURA trial was a randomized, double-blind, placebo-controlled, phase 3 study that evaluated the survival benefits of adjuvant osimertinib, an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI), in the treatment of patients with completely resected EGFR-mutated stage IB-IIIA non-small cell lung cancer (NSCLC).1 A total of 682 adult patients with completely resected stage IB, II, IIIA NSCLC were recruited and randomized 1:1 to receive either osimertinib 80mg daily or placebo for 3 years until disease recurrence or treatment completion.1 The primary endpoint of the ADAURA trial was disease-free survival (DFS) in stage II-IIIA patients.1 Overall survival (OS) is one of the key secondary endpoints of the ADAURA trial.1
Published in 2020, the primary analysis of the ADAURA trial showed that adjuvant osimertinib therapy could induce a significantly longer DFS in comparison to placebo in patients with EGFR-mutated stage IB-IIIA NSCLC (HR=0.20; 99.12% CI: 0.14-0.30; p<0.0001).1 The result was confirmed in a subsequent DFS analysis conducted in 2023 (HR=0.27; 95% CI: 0.21-0.34).1 The recent OS analysis of ADAURA has demonstrated the OS benefits of adjuvant osimertinib among patients with EGFR-mutated stage IB-IIIA NSCLC.1 Notably, ADAURA is the only phase 3 trial to date that successfully translated the DFS benefit of EGFR-TKI into significant OS benefits, thereby reinforcing osimertinib as a standard adjuvant therapy for patients with resected EGFR-mutated stage IB-IIIA NSCLC.1
FINDINGS
|
Key secondary endpoints: |
|
|
|
|
|
|
Safety: |
|
|
|
"ADAURA was the first global phase 3 study that demonstrated statistically significant and clinically meaningful OS benefits with targeted treatment in this patient population, reinforcing adjuvant osimertinib as a standard of care for patients with resected EGFR-mutated stage IB-IIIA NSCLC"
Dr. Roy S. Herbst
Yale School of Medicine and Yale Cancer Center,
New Haven, Connecticut, United States

