EXPERT INSIGHT
The potential new first-line mNSCLC treatment regardless of PD-L1: Durvalumab + tremelimumab + chemotherapy improved survival
Over the past decades, treatment options in advanced non-small cell lung cancer (NSCLC) patients without oncogenic drivers have been limited to cytotoxic chemotherapies with poor survival outcomes.1 Although patients’ overall survival (OS) has been prolonged with the current standard of care (SoC) (i.e. pembrolizumab with or without chemotherapy) in recent years, the clinical outcomes are still suboptimal.2,3 Dual immunotherapy, which brought substantial survival improvements across multiple malignancies such as advanced melanoma, sheds light on the further advance of metastatic NSCLC (without driver mutations) management.4 The combination of nivolumab (NIVO) and ipilimumab (IPI) with or without chemotherapy has demonstrated superior survival benefits in these patients, leading to the regulatory approval from the United States (US) Food and Drug Administration (FDA).5,6 More recently, the efficacy of durvalumab and tremelimumab plus chemotherapy (D + T + CT) in treatment-naïve metastatic NSCLC patients has also been evaluated in the POSEIDON trial.7 In a webinar organized by the Hong Kong Precision Oncology Society, Dr. Melissa L. Johnson presented the encouraging data from POSEIDON and discussed the latest advances of immunotherapy in metastatic NSCLC. Dr. Au, Siu-Kie Joseph also shared his expert insights on the new POSEIDON data and discussed their impacts on the local clinical practice in an interview with Omnihealth Practice.
Unmet needs in metastatic NSCLC patients despite current SoC
Pembrolizumab with or without chemotherapy, depending on patients’ tumor programmed death-ligand 1 (PD-L1) expression level, is currently considered by the National Comprehensive Cancer Network (NCCN) as one of the preferred systemic therapies for metastatic NSCLC (without driver mutations).8 The efficacy of pembrolizumab was demonstrated in a number of clinical trials, including the KEYNOTE-024 and the KEYNOTE-189. Yet, the latest update of KEYNOTE-024 found that the 5-year OS rate for patients on pembrolizumab monotherapy was only 31.9%.3 “That means the majority of patients were not alive 5 years later with anti-programmed death 1 (PD-1) therapy alone,” Dr. Johnson said. On the other hand, the 4-year update of KEYNOTE-189 indicated that only about 30% of patients on pembrolizumab + chemotherapy survived at 3 years (3-year OS rate: 31.3%), with the greatest survival benefits observed in patients with PD-L1 ≥50% (3-year OS rate: 43.7%) and less notable benefits among those with PD-L1 <50% (3-year OS rate: 23.3-28.3%).2 Similar variances in progression-free survival (PFS) were observed across patients with different PD-L1 levels.2 These findings reflected that there were still unmet needs among NSCLC patients, particularly those with PD-L1 <50%. “There is more work to do,” Dr. Johnson mentioned.
Dual PD-L1 and CTLA-4 blockades’ long-term OS improvements in melanoma showed promise in NSCLC
The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1 are the key immune checkpoints that inhibit T cell functions.9 CTLA-4 regulates the proliferation of T cells early in the lymph nodes, whereas PD-1 suppresses T cells later in the peripheral tissues (e.g. tumor sites).9 Since tumor cells are capable of developing ways to evade the host immune system by taking advantage of these immune checkpoints, simultaneous inhibition of CTLA-4 and PD-1 (i.e. dual pathway blockade) was believed to have a synergistic effect in obtaining a larger and longer-lasting antitumor immune response (figure 1).9
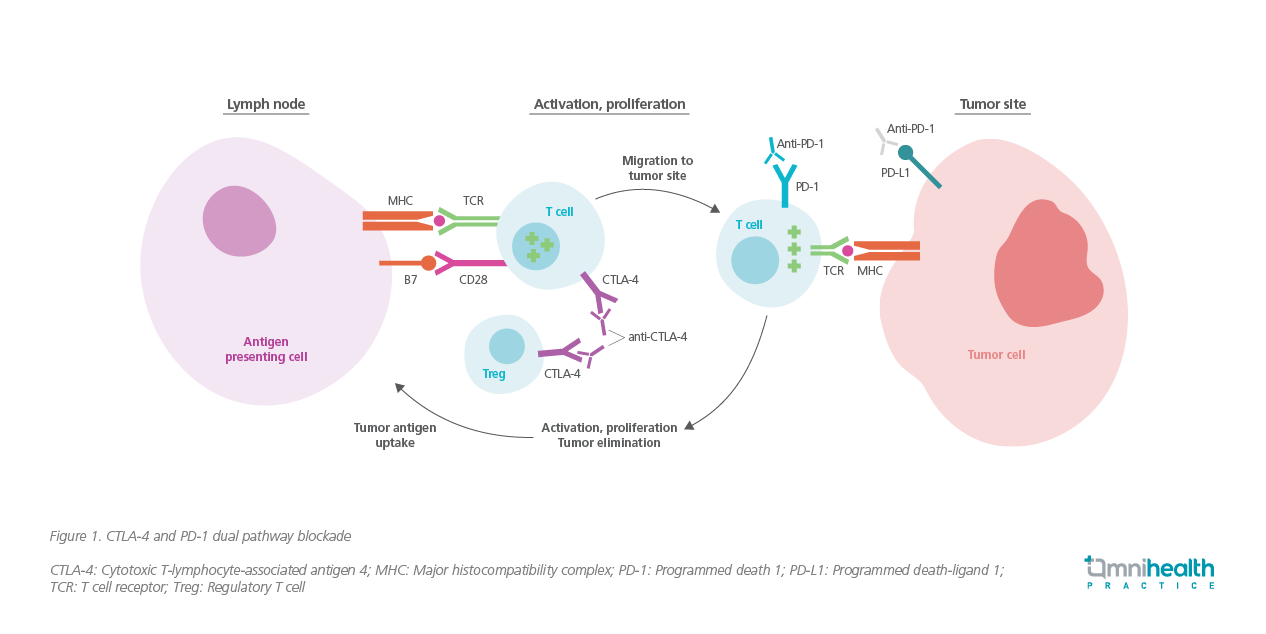
The novel dual immunotherapy was first adopted in the management of advanced melanoma, with the remarkable efficacy of NIVO + IPI demonstrated in CheckMate 067.4 With a minimum follow-up of 6.5 years, the median OS of patients treated with NIVO + IPI reached 72.1 months vs. 36.9 months with NIVO alone or 19.9 months with IPI alone.4 The impressive and encouraging results from the melanoma trial shed light on the further improvement of the survival outcomes with dual immunotherapy in NSCLC patients.
NIVO + IPI: The first approved dual immunotherapy in metastatic NSCLC with long-term survival benefits
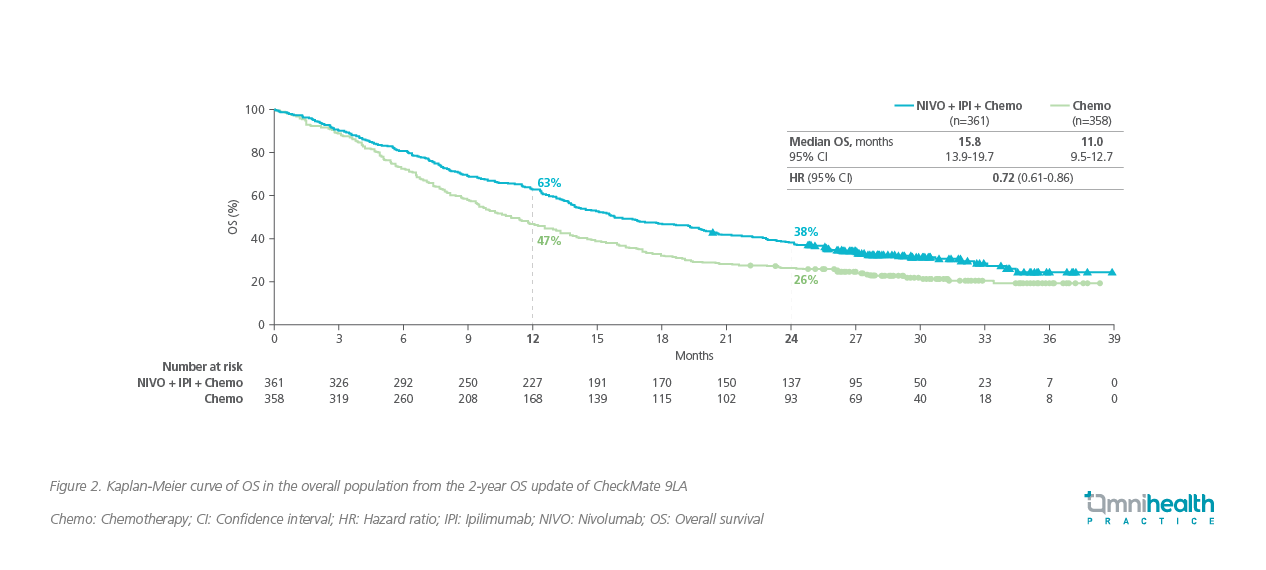
NIVO + IPI is the first approved dual immunotherapy for the treatment of advanced NSCLC. The long-term survival benefits of NIVO + IPI with or without chemotherapy in patients with stage IV or recurrent NSCLC were shown in CheckMate 9LA and CheckMate 227, respectively.5,10 With a median follow-up of 54.8 months in CheckMate 227, 29% of patients with PD-L1 ≥1% on NIVO + IPI remained alive at 4 years vs. 18% with with the chemotherapy arm.10 Among patients with PD-L1 <1%, 24% were alive at 4 years with NIVO + IPI vs. 10% with chemotherapy alone.10 In CheckMate 9LA (median follow-up: 30.7 months), the median OS of patients on NIVO + IPI with chemotherapy was 15.8 months vs. 11.0 months with chemotherapy alone (HR=0.72; 95% CI: 0.61-0.86) (figure 2).5 The 2-year OS rate was 38% with the triplet therapy vs. 26% with chemotherapy.5 Both trials achieved good long-term survival curves which plateaued out and were maintained over time.5,10
POSEIDON trial demonstrated survival benefits as a new dual checkpoint alternative in metastatic NSCLC
POSEIDON was a global, randomized, open-label, phase 3 trial that evaluated the safety and efficacy of D + T + CT as first-line therapy in treatment-naïve patients with stage IV NSCLC without driver mutations.7 A total of 1,013 eligible patients were enrolled and randomized 1:1:1 to receive durvalumab 1,500mg + tremelimumab 75mg with chemotherapy every 3 weeks for 4 cycles, or durvalumab 1,500mg with chemotherapy every 3 weeks for 4 cycles, or platinum-based chemotherapy every 3 weeks for up to 6 cycles at the treatment initiation phase.7 At the maintenance phase, patients were treated until disease progression with durvalumab 1,500mg every 4 weeks (additional 1 dose of tremelimumab 75mg at week 16 only and with pemetrexed if non-squamous histology); durvalumab 1,500mg every 4 weeks ( with pemetrexed if non-squamous histology), or pemetrexed alone (if non-squamous histology) respectively (figure 3).7

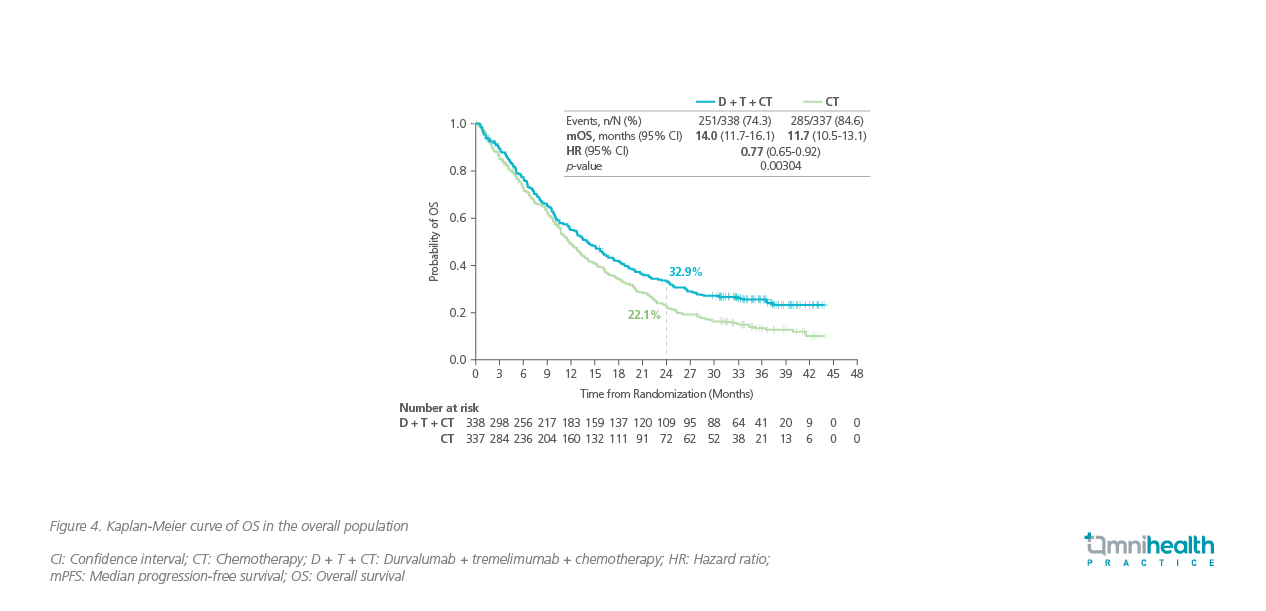
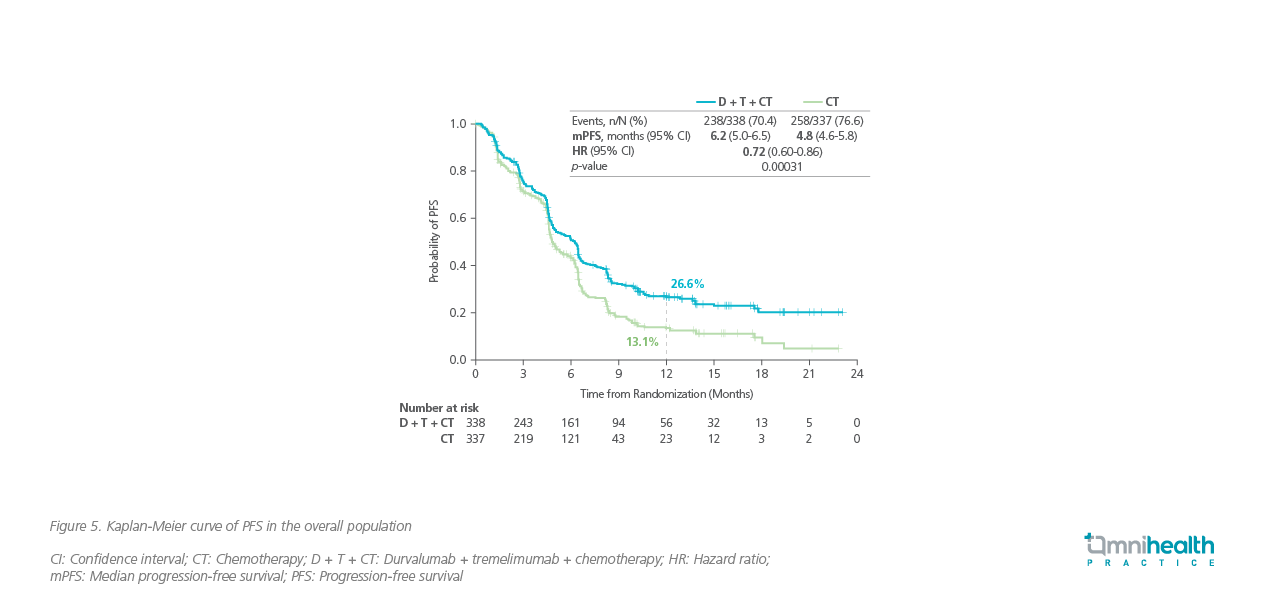
Consistent survival benefits were observed in patients treated with D + T + CT in POSEIDON, regardless of tumor PD-L1 expression level.7 With a median follow-up of 34.9 months, patients on the triplet therapy group achieved a significantly longer median OS of 14.0 months vs. 11.7 months in the chemotherapy group (HR=0.77; 95% CI: 0.65-0.92; p<0.00304) (figure 4).7 Similar to CheckMate 227 and CheckMate 9LA, the tail of the survival curve in POSEIDON began to separate and plateaued out over time.7 The risk reductions in overall mortality by D + T + CT among patients with PD-L1 <1% and ≥1% were similar (HR=0.77 vs. 0.76).7 The median PFS by the BICR was also significantly longer with the triple therapy arm compared with chemotherapy alone [6.2 months vs. 4.8 months (HR=0.72; 95% CI: 0.60-0.86; p<0.00031)] (figure 5).7
The addition of tremelimumab into the treatment of durvalumab + chemotherapy (D + CT) resulted in a slight increase in the incidence rate of any-grade treatment-related adverse events (TRAEs) (92.7% vs. 88.6%).7 The number of reported serious adverse events (AEs) associated with D + T + CT were also higher (27.6%) than the D + CT group (19.5%) and the chemotherapy group (17.7%).7 The rates of AEs which led to treatment discontinuation in the D + T + CT and D + CT groups were similar (15.5% vs. 14.1%).7 “This suggests that we have become more comfortable and faster to identify the immune-mediated AEs and intervene early to prevent serious consequences,” Dr. Johnson stated.
Local expert opinions on POSEIDON
In an interview with Omnihealth Practice, Dr. Au, Siu-Kie Joseph shared his insights on the new POSEIDON data and discussed its implications on the local practice. Dr. Au mentioned that the treatment decisions for metastatic NSCLC patients (without driver mutations) are currently based on the level of tumor PD-L1 expression and the tempo of disease progression mainly.8 Pembrolizumab monotherapy is recommended for patients with PD-L1 ≥50%; however, if the patients have rapidly progressive disease, chemotherapy can be administered concomitantly.8 Except for oligometastatic patients who can be treated effectively with localized therapies, achieving long-term survival among most metastatic NSCLC patients, especially those with PD-L1 <50%, remains a challenge despite the current standard of treatment. CheckMate 227 and CheckMate 9LA showed a certain level of treatment success with the dual immunotherapy of NIVO + IPI with or without chemotherapy.5,10 The results of POSEIDON with D + T + CT were equally promising and provided a new dual checkpoint option with 5 convenient doses of tremelimumab (vs. continuous administration of IPI until disease progression) for patients.7 It may be misleading to look at the median OS data only in the assessment of survival benefits. As shown in these 3 trials, the tail of the survival curves also provided valuable information about patients’ long-term survivorship.5-7,10 In fact, the plateauing of the tails with dual CTLA-4 and PD-1 blockade was commonly seen in different trials across multiple malignancies.9
As the President of the Hong Kong Precision Oncology Society, Dr. Au advocated the precision medicine approach not just for targeted therapies but for immunotherapies as well. Although a completely personalized approach in oncology is still remote, the success of classifying non-small cell lung cancer into different molecular subtypes for different targeted therapies have raised much optimism. For immunotherapy, this approach has been less successful, and currently, the tumor PD-L1 expression level is the major predictive biomarker.8 Nevertheless, this indicator seemed to have lost its predictability with dual immunotherapy as observed in the survival benefits demonstrated in all patients regardless of the PD-L1 levels.5-7,10
“Since cure is unlikely in metastatic NSCLC setting, most patients are treated with palliative care with the goals of prolonging survival and preserving their quality of life (QoL),” Dr. Au stressed. Although dual immunotherapies demonstrated good clinical efficacies, the trials also showed increased rates of AEs, particularly immune-mediated AEs, which may compromise patients’ QoL.7
“The early detection of serious AEs and timely intervention are the effective measures and key to preventing permanent and irreversible damage in patients,” Dr. Au added. However, this could be challenging during the Coronavirus Disease 2019 (COVID-19) pandemic. It is highly recommended to set up a patient self-report system, such as creating a hotline or web-based system for patients to report symptoms using a standardized AE reporting questionnaire so that development of serious side effects can be detected early.11 Caregivers should also follow up frequently with patients via telephone proactively.12
Dr. Au summarized that the dual pathway blockade has been proven as an effective strategy to tackle tumor cells’ mechanisms to evade the host immunity for improving survival in metastatic NSCLC patients. The new data from POSEIDON and CheckMate 9LA were particularly beneficial to patients with low PD-L1 levels whose medical needs were previously unmet.
Conclusion
In summary, the promising data of dual immunotherapy are changing the treatment paradigm of advanced or metastatic NSCLC, with the POSEIDON trial providing a new potential first-line treatment option (D + T + CT) for patients. The tumor PD-L1 expression level may have lost its predictability in patients’ treatment outcomes when anti-CTLA-4 is added to anti-PD-L1 therapy. Despite the long-term survival benefits with the dual pathway blockade, increased AEs were reported. Hence, the early detection and intervention with close patient monitoring are the effective measures to be considered and key to preventing permanent and irreversible damage, as well as preserving patients’ QoL.

