CASE REVIEW
Adjuvant osimertinib and multidisciplinary-team approach in early-stage NSCLC management: A local case sharing
Non-small cell lung cancer (NSCLC) accounts for 85%of all types of lung cancer and is the most common cancer in Hong Kong with a total of 5,575 cases in 2019.1,2 Approximately 30% of NSCLC patients are diagnosed as early stage, and surgical resection is the preferred treatment option for possibly complete removal of the tumor mass with a curative intent.1 However, studies found that the relapse rates after surgery remain high, despite the adoption of adjuvant chemotherapy.1 Recent advances in precision medicine have brought in novel adjuvant treatment options, particularly adjuvant osimertinib for patients with epidermal growth factor receptor (EGFR)-sensitizing and EGFR T790M resistance mutations.1 The phase 3 ADAURA study has shown that adjuvant osimertinib could significantly reduce the risk of disease recurrence and prolong disease-free survival (DFS) among patients with EGFR mutations. More importantly, due to the complexity of disease and the need for multiplicity of treatment options, collaboration among specialists across different disciplines, especially oncologists and thoracic surgeons, is crucial for achieving better treatment outcomes. In an interview with Omnihealth Practice, Dr. Suen, Hon-Chidiscussed the importance of multidisciplinary management of early-stage NSCLC and the key considerations of a thoracics surgeon in referring patients for emerging adjuvant treatments.
Background
Surgery is the gold standard for early-stage NSCLC management
The incidence of early-stage NSCLC is anticipated to rise with the rapidly aging population and the increased screening for high-risk patients.3 Currently, about 30% of NSCLC patients present with stage I and II diseases that are functionally operable and have a 5-year survival rate of 47%-82%.3 According to the latest National Comprehensive CancerNetwork (NCCN) and the European Society of Medical Oncology (ESMO)guidelines, surgery remains the standard of care and provides the best chance of cure for this group of patients.4,5 Surgical options include complete surgical resection with lobectomy and conservative resections with sublobar resection, sleeve resection and minimally invasive techniques such as video-assisted thoracic surgery (VATS).3 The extent of resection and the precise surgical approach mainly depends on patients’ cardiopulmonary reserve and other important clinical considerations, such as the natural history of disease and the risks and benefits of each treatment option.3 Lobectomy has been the gold standard for resection of early-stage NSCLC for decades, while sublobar resection is only reserved for select patients with poor pulmonary reserve or other major comorbidities contraindicating lobectomy, since it is associated higher risk of death and a three-fold increased risk for local relapses.6 In recent years, however, new high-quality data from randomized trials have emerged, showing that patients with stage IA NSCLC (tumor diameter ≤2cm;consolidation-to-tumor ratio >0.5) who had received sublobar resection/segmentectomy achieved a longer or similar overall survival (OS) and a comparable DFS when compared with lobectomy.6,7 The findings suggest that segmentectomy and sublobar resection can be effective alternatives to lobectomy in this patient population.6,7
Limited values of adjuvant chemotherapy for preventing disease recurrence
Based on the results of several clinical trials, the current NCCN guidelines recommend post-operative platinum-based chemotherapy among patients with early-stage resectable NSCLC for preventing disease recurrence and obtaining survival benefits.5 However, clinical benefits from adjuvant chemotherapy are de facto limited.5 Among patients with completely resected early-stage NSCLC, adjuvant chemotherapy only reduced the risk of death and disease recurrence by 14% (HR=0.86; 95% CI: 0.76-0.98; p<0.03) and 17% (HR=0.83; 95% CI: 0.74-0.94; p<0.003), respectively.8 Despite post-operative chemotherapy (vs. observation), the prolonged5-year survival rate of patients was still less than 50%.8 Upon a longer follow-up of 7.5 years, there were more deaths in the chemotherapy group beyond 5 years of follow-up, indicating that the benefits of chemotherapy waned over time.9 Furthermore, several real-world studies pointed out that approximately 48%-57% of patients with resectable early-stage NSCLC received chemotherapy after surgery in clinical practice.10,11 Nevertheless, disease recurrence rate remained high among patients with stage IB-IIIA NSCLC, in spite of the availability of chemotherapy, emphasizing the need for more effective systemic adjuvant therapies in this population.10
Improved DFS with adjuvant osimertinib for completely resected EGFR-mutated, early-stage NSCLC
Osimertinib is a third-generation, irreversible, oral EGFR tyrosine kinase inhibitor (TKI) that selectively and potently inhibits bothEGFR T790M and EGFR TKI-sensitizing resistance mutations.12 It was first recommended for treating patients with EGFR-mutated advanced NSCLC and acquired T790M mutation after disease progression on other first-line EGFR TKIs.12 More recently, osimertinib has also been shown to have remarkable survival benefits in the adjuvant setting.13 ADAURA is a double-blind, phase 3 trial which aimed to assess the safety and efficacy of osimertinib among completely resected stages IB to IIIA EGFR mutation-positive NSCLC patients.13 Eligible patients (n=682) were randomized 1:1 to receive either adjuvant osimertinib 80mg daily or placebo for 3 years.13Patients with American Joint Committee on Cancer (AJCC) stage IB and II NSCLC accounted for 32% and 34% of the entire population, respectively.13 With a median follow-up of 44.2 months for patients on osimertinib and 19.6 months for patients on placebo (data-cutoff: April 11, 2022), adjuvant osimertinib demonstrated a significant reduction in the risk of disease recurrence vs. placebo among the NSCLC patients (HR=0.27; 95% CI: 0.21-0.34) (figure 1).14 About 73%of patients treated with adjuvant osimertinib were alive and remained disease-free after 4 years of treatment vs. only 38% in the placebo group.14 The DFS benefits were consistent across patients at different stages of disease (stage IB: HR=0.41; 95% CI: 0.23-0.69; stage II-IIIA: HR=0.23; 95% CI: 0.18-0.30).14

In ADAURA, about 60% (410/682) of patients received adjuvant chemotherapy for a median duration of 4 cycles.12 The sub-analysis of ADAURA revealed that the DFS benefits favored osimertinib over placebo among patients with (HR=0.16; 95% CI: 0.1-0.26) or without(HR=0.23; 95% CI: 0.13-0.40) adjuvant chemotherapy, regardless of the disease stages (figure 2 and 3, stage II disease only).12
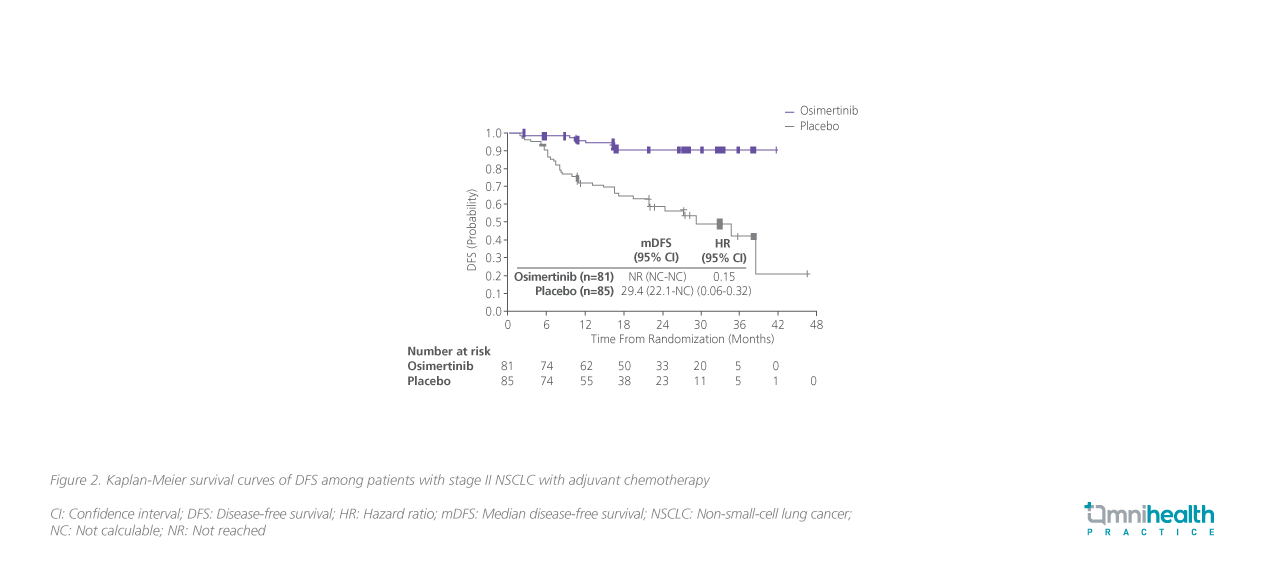
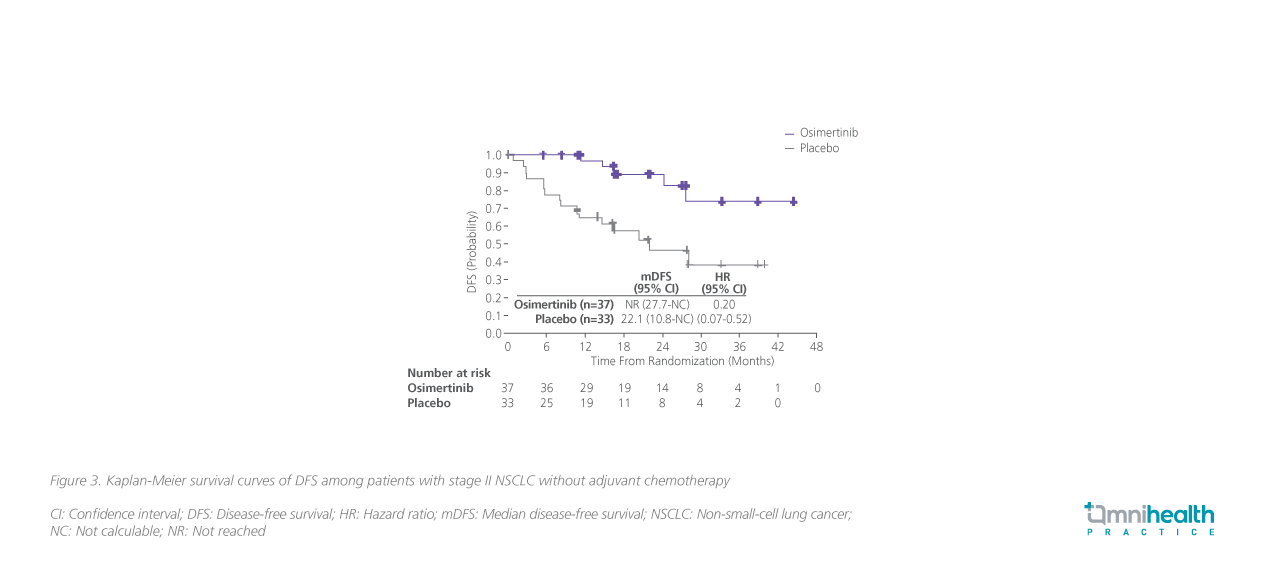
Based on the positive results of the ADAURA trial, the latest NCCNguidelines recommend osimertinib as an adjuvant therapy option for eligible patients with completed resected stage IB to IIIA EGFRmutation-positive NSCLC who have previously received adjuvant chemotherapy or are ineligible for platinum-based chemotherapy.5“Given the clinical data showing improved survival benefits with adjuvant osimertinib, we should consider and discuss the use of the drug in the multidisciplinary team (MDT) meetings and with the patients,” Dr. Suen said.
Case sharing
In 2018, a 67-year-old female presented to her primary care physician with intermittent cough and shortness of breath on exertion. She was a never-smoker without any past medical history. A chest X-ray revealed a shadow over the anterior segment of her upper lobe of the right lung.
In April 2020, the patient was referred to an oncologist. Positron emission tomography (PET)/computed tomography (CT) scan showed an enlarging right upper lobe shadow, confirming a lung lesion that was highly suggestive of lung cancer. The brain magnetic resonance imaging (MRI) was negative. The tumor, node, metastasis (TNM)staging was T2bN0M0 (stage IIA), and the patient was subsequently referred to thoracic surgeons.
In May 2020, a series of surgical procedures, including fiberoptic bronchoscopy (FOB), uniportal right VAT, right upper lobectomy, and lymph node resection, were performed. Molecular testing on the surgical samples revealed EGFR exon 21 L858R mutation. Since chemotherapy was not suitable for this patient, adjuvant osimertinib was started right after the surgery to reduce the risk of disease recurrence.
As of April 2023, the patient has been on adjuvant osimertinib for almost 3 years. No sign of disease recurrent was noted, and the treatment was well tolerated by the patient.
Discussion
Owing to the underlying biological diversity and the complexity of the patient population, lung cancer management is no doubt an immense challenge.15 According to the latest international guidelines on NSCLC, active treatment options include surgery (e.g., lobectomy), radiation therapy (e.g., stereotactic ablative radiotherapy), platinum-based chemotherapies, targeted therapies (e.g., osimertinib), and immunotherapy(e.g., durvalumab).4,5 Generally speaking, the optimal management of NSCLC patients involves the use of ≥2 modalities.4,5,11 “Apparently, surgeons are not drug experts. We surely need to consult oncologists when there are benefits with pharmacological intervention; likewise, when oncologists consider surgery as beneficial to patients, referring them to thoracic surgeons is highly encouraged,” Dr. Suen asserted. Close collaboration among multiple specialists, especially oncologists and thoracic surgeons, is therefore of paramount importance to develop an optimal NSCLC management plan.
In fact, international guidelines have already recognized the importance of multidisciplinary cancer care and recommended that the diagnosis and treatment of lung cancer should be based on the consensus of multiple specialists.8 Studies revealed that patients managed by the MDT approach were more likely to have better clinical outcomes.15 For instance, a large cohort study with >4,000 patients was conducted to evaluate the impact of MDT management on survival outcomes among patients of all-stage NSCLC.16 A significant improvement was observed in all-stage NSCLC patients at 5 years vs. the non-MDT approach (HR=0.65; 95% CI: 0.54-0.77).16 In addition, patients managed by MDT are more likely to receive all treatment modalities.15 A study found that the establishment of a teleconferenced MDT meeting led to a 30% increase in the surgery rates, presumably due to a more direct referral pathway by oncologists to surgeons.17 The likelihood of surgery for stage I/II lung cancer patients was almost 3 times higher with regular attendance at the MDT meeting(OR=2.9; 95% CI: 1.3-6.8).18 The MDT meeting also resulted in 97% of treatment plans in concordance with the NCCN guidelines vs. 81% before the MDT was set up.19 More importantly, high-level of distress has been reported in patients with lung cancer, and the MDT meeting might provide an opportunity for assessing patients' quality of life (QoL) and screening for patients’ psychological concerns, which could be crucial to allow timely intervention and rapid referral, when necessary.15
Conclusion
In summary, early-stage NSCLC is a complicated disease that requires MDT efforts and novel treatment options such as adjuvant osimertinib for achieving better outcomes. The DFS benefits with adjuvant osimertinib reported in ADAURA are undeniably encouraging and give hope to patients for a better chance of cure.

