CONFERENCE UPDATE: ASCO 2024
First-line lorlatinib continues to show PFS benefit over crizotinib in patients with advanced ALK+ NSCLC: 5-year follow-up of the CROWN study
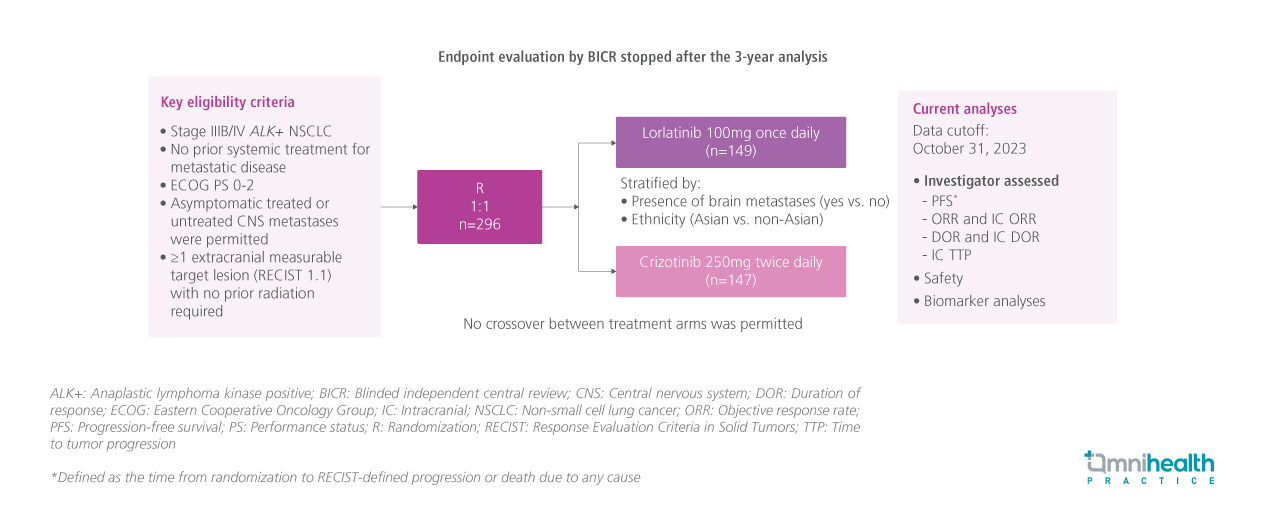
STUDY DESIGN
Lorlatinib is a brain-penetrant third-generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI) which covers a broader ALK resistance profile than second-generation ALK TKIs.1 It has previously demonstrated improved progression-free survival (PFS) and intracranial (IC) activity compared to crizotinib in treatment-naïve patients with advanced ALK+ non-small cell lung cancer (NSCLC) in the ongoing randomized phase 3 CROWN study.1
In the CROWN study, a total of 296 treatment-naïve patients with advanced ALK+ NSCLC were randomized to receive lorlatinib 100mg once daily (QD) (n=149) or crizotinib 250mg twice daily (BID) (n=147).1 At approximately 3 years of follow-up, median PFS by blinded independent central review (BICR) was not reached (NR) in the lorlatinib arm and 0.3 months in the crizotinib arm (HR=0.27; 95% CI: 0.18-0.39).1
This post-hoc analysis evaluated the longer-term (5-year follow-up) efficacy and safety outcomes of lorlatinib versus crizotinib in the CROWN study.1 Key efficacy outcomes presented at the meeting included investigator-assessed PFS and time to IC tumor progression which were stratified by patients’ ethnicity (Asian or non-Asian) and the presence of brain metastases, gender, age (<65 or ≥65 years) and smoking status.1 Brain tumor assessments were conducted using brain magnetic resonance imaging (MRI) every 8 weeks.1 Biomarker analyses were also conducted by evaluating the circulating tumor deoxyribonucleic acid (ctDNA) to determine potential resistance mechanisms.1
FINDINGS
| Efficacy outcomes |
|
|
|
|
|
|
|
|
|
Safety: |
|
|
|
|

