CONFERENCE UPDATES: ASCO 2023
Sotorasib demonstrates consistent clinical benefits in KRAS G12C-mutation advanced NSCLC regardless of genomic co-alterations: Subgroup analysis of CodeBreaK 200
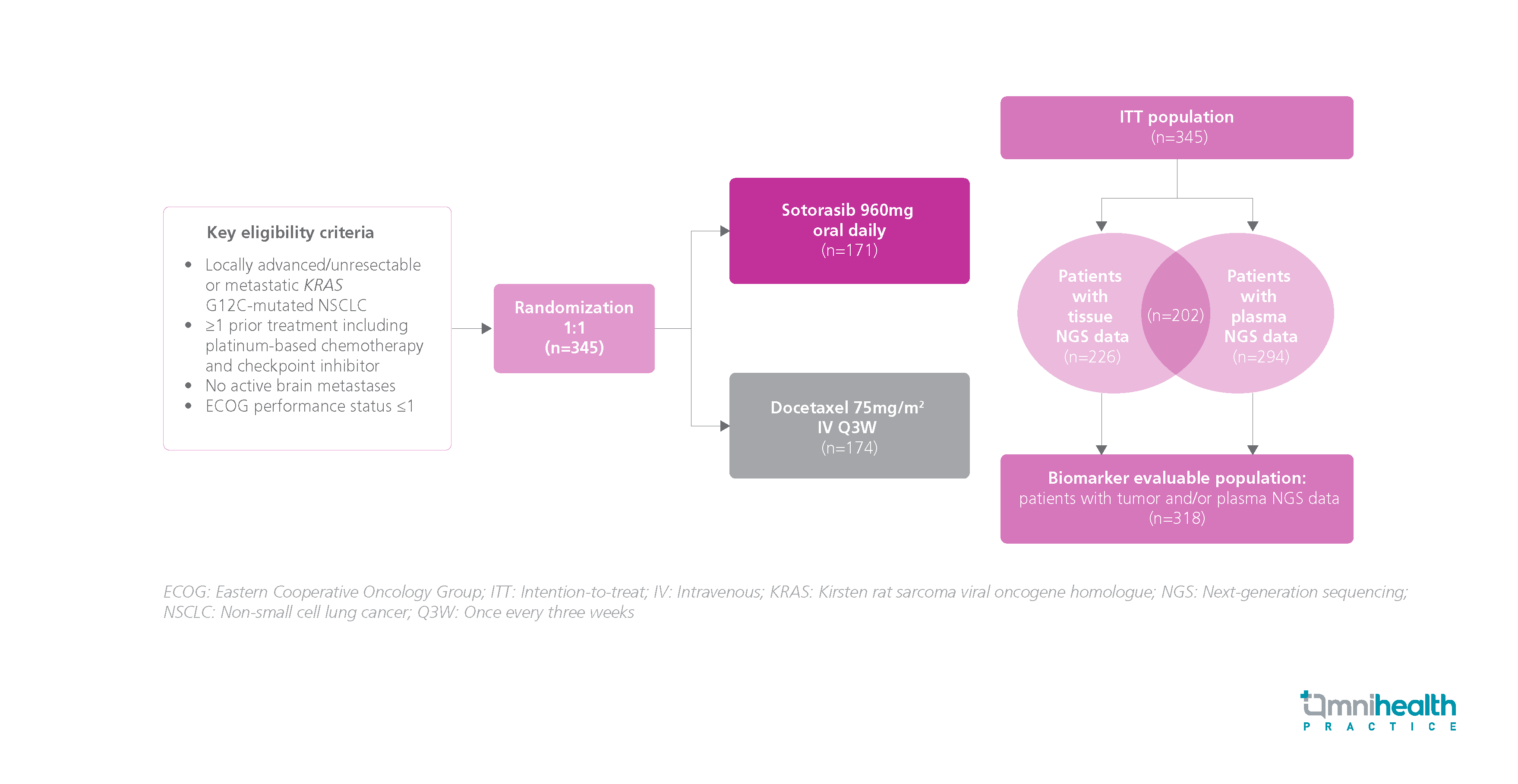
STUDY DESIGN
The CodeBreaK 200 trial was the first randomized, phase 3 trial that compared the efficacy between sotorasib and docetaxel in patients with Kirsten rat sarcoma viral oncogene homolog (KRAS) G12C-mutated advanced non-small cell lung cancer (NSCLC).1 A cohort of 345 adults with locally advanced or metastatic KRAS G12C-mutated advanced NSCLC who had received prior platinum-based chemotherapy and had no active brain metastases were enrolled.1 Participant were randomized 1:1 to either receive a daily oral dose of sotorasib 960mg or an intravenous (IV) injection of docetaxel 75mg/m2 once every 3 weeks (Q3W).1 The primary endpoint of CodeBreaK 200 was progression-free survival (PFS), whereas the secondary endpoints included the overall response rate (ORR), genomic co-alterations, and programmed death-ligand 1 (PD-L1) protein levels.1 In the primary analysis, sotorasib demonstrated superior PFS (HR=0.66; 95% CI: 0.51-0.86; p=0.002) and ORR in providing a significantly longer PFS for patients with KRAS G12C-mutated NSCLC when compared with docetaxel.1
In this exploratory biomarker analysis, the baseline tissue and/or plasma samples from the biomarker-evaluable population (n=318) were analyzed for key genomic alterations using central targeted next-generation sequencing (NGS).1 A total of 13 types of key co-alterations were identified in this patient population, including tumor protein 53 (TP53), serine/threonine kinase 11 (STK11), kelch-like ECH-associated protein 1 (KEAP1), etc.1 Also, programed cell death ligand-1 (PD-L1) was assessed.1
FINDINGS
| Exploratory endpoints: |
|
|
|
- TP53 (Wild-type: HR=0.48; 95% CI: 0.30-0.75, altered: HR=0.83; 95% CI: 0.58-1.18) |
|
- STK11 (Wild-type: HR=0.65; 95% CI: 0.46-0.92, altered: HR=0.68: 95% CI: 0.45-1.05) |
|
- KEAP1 (Wild-type: HR=0.62; 95% CI: 0.45-0.84, altered: HR=0.84; 95% CI: 0.48-1.47) |
|
|
|
|
- <1% (HR=0.66; 95% CI: 0.41-1.06; p=0.06) |
|
- ≥1% and <50% (HR=0.61; 95% CI: 0.39-0.96; p=0.03) |
|
- ≥50% (HR=0.74; 95% CI: 0.44-1.23; p=0.14) |
“Sotorasib has demonstrated consistent clinical benefits across multiple key co-alteration patient subgroups of KRAS G12C-mutated NSCLC, regardless of the PD-L1 expression status”
Dr. Ferdinandos Skoulidis
University of Texas MD Anderson Cancer Center,
Houston, Texas, United States

