CASE REVIEW
Using adjuvant osimertinib to treat resected EGFR mutationpositive NSCLC in early stages: A local case sharing
Accounted for 15.4% of new cancer cases in 2018, lung cancer is one of the most common cancers in Hong Kong, with around 30% of patients having resectable non-small cell lung cancer (NSCLC).1,2 While adjuvant chemotherapy is the current postoperative standard of care, this treatment could only reduce the risk of disease recurrence or death by 16%.2 Recently, the ADAURA trial demonstrated that osimertinib, when utilized as an adjuvant therapy with or without chemotherapy, could prolong the disease-free survival (DFS) of patients with resected stage IB to IIIA epidermal growth factor receptor (EGFR) mutation positive NSCLC.2 In a recent interview with Omnihealth Practice, Dr. Tsang, Wai-Kong Maverick, shared a local patient case with resected stage IB EGFR mutation-positive lung adenocarcinoma who was well-tolerated to the postoperative adjuvant osimertinib without chemotherapy for 10 months.
Background
The 5-year survival rates of NSCLC is only 50% in patients with stage II disease and less than 30% in stage III patients.2 As lung cancer can progress rapidly, early intervention is preferred when the disease is still in its early stage.
Osimertinib is a third generation EGFR tyrosine kinase inhibitor (EGFR-TKI) that inhibits both the EGFR-TKI sensitizing and EGFR p.Thr790Met resistance mutations, and is associated with benefits in patients with NSCLC and central nervous system metastasis.2 With the recent ADAURA study demonstrating a significant survival benefit of osimertinib in patients with stage IB to stage IIIA resected NSCLC, the National Comprehensive Cancer Network (NCCN) guidelines version 1.2022 recommend either observation or chemotherapy for high-risk patients with stage IB and IIA NSCLC with negative margins, and consider osimertinib for those who are positive for EGFR mutations, received adjuvant chemotherapy or are ineligible to receive platinum-based chemotherapy.3 In addition, Osimertinib with chemotherapy should be considered for patients with negative margins and stage IIB and III NSCLC.3 A local patient case is shared below to demonstrate the adoption of osimertinib in the disease control of patients with early stage resected NSCLC.
Case report
A 52-year-old, non-smoking female was diagnosed with stage IB lung adenocarcinoma with EGFR exon 21 codon p.Leu858Arg (L858R) mutation which involved visceral pleural and lymphovascular invasion (LVI), expressing a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) of 5%. Lobectomy of the right upper lobe (RUL) with clear resection margins was undergone in January 2021, with postoperative obstructive narrowed right middle lobe (RML) atelectasis.
Given her high risk of disease recurrence (presence of visceral pleural involvement and LVI) and EGFR mutations, the patient was prescribed with a 3-year adjuvant osimertinib treatment and was initiated in February 2021. Adjuvant chemotherapy was also advised but refused by the patient due to concerns about the side effects.
After 9 months of osimertinib only treatment, the patient’s disease state was well under control with no suspected lesions shown in chest X-ray, despite an elevated right hemidiaphragm. The treatment was generally well-tolerated. Serial electrocardiograms (ECGs) showed normal QTc interval. There was a transient grade 1 neutropenia with the lowest neutrophil count of 1.65 x 109/L. The latest blood test showed normal complete blood picture (CBP), liver function test, and carcinoembryonic antigen (CEA) (0.7ng/mL).
Osimertinib-related toxicities included mild skin rashes (grade 1) at both legs and mild neutropenia (grade 1). Apart from having a mild coughing, the patient had osimertinib-related diarrhoea (grade 1) which was controlled with probiotics. No dosage adjustment was required and the continued adoption of osimertinib was planned for the next 3 years. CEA and ECG will be performed in every 3 months to consistently monitor her NSCLC management with osimertinib alone.
Discussion
In the previous phase 3 FLAURA trial, osimertinib demonstrated superior progression-free survival (PFS) and overall survival (OS) benefits to gefitinib or erlotinib in the previously untreated patients with EGFR mutation-positive (Ex19del or L858R) advanced NSCLC.2 About 45% of patients with stage IB disease and up to 76% with stage III disease will experience disease recurrence or mortality in 5 years after surgery, regardless of the postoperative chemotherapy. Therefore, the ADAURA trial was conducted to evaluate whether the benefits of adjuvant osimertinib can be extended to these patients.2,4
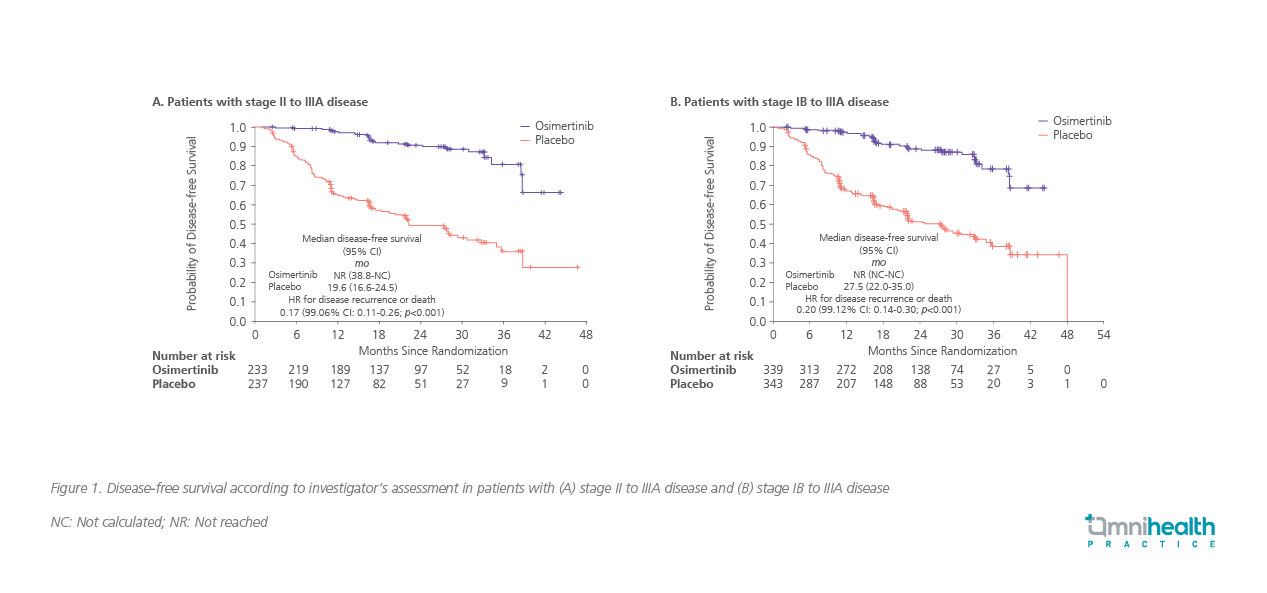
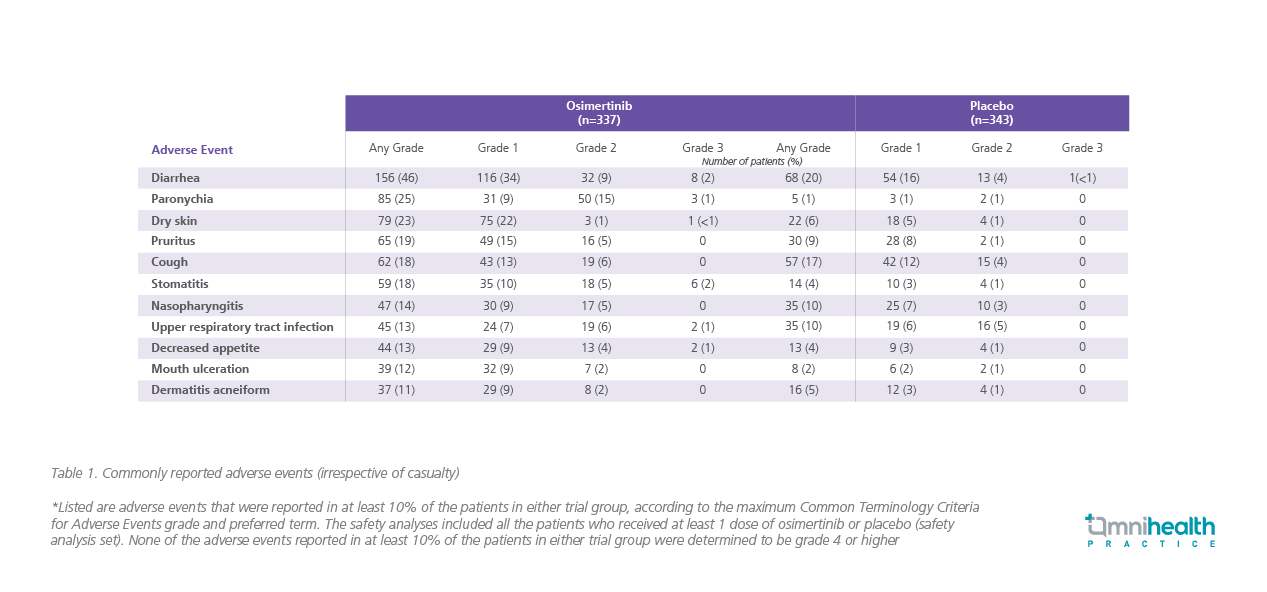
In the phase 3, double-blinded, randomized ADAURA trial (n=682) conducted in patients with completely resected EGFR mutation-positive NSCLC, the DFS among patients with stage IB to IIIA disease were demonstrated with 3-years of osimertinib (80mg once daily) vs. placebo.2 The median follow-up for DFS was 22.1 months in the osimertinib group and 14.9 months in the placebo group.2 Among the 470 patients with stage II to IIIA disease, the percentage of patients achieving DFS at 24 months was 90% in the osimertinib group vs. 44% in the placebo group (HR=0.17; 99.06% CI: 0.11-0.26; p<0.001; figure 1A).2 Similarly, in the overall population (n=682), 89% of patients receiving osimertinib were alive and disease-free at 24 months, vs. 52% in the placebo group (HR=0.20; 99.12% CI: 0.14-0.30; p<0.001; figure 1B).2 The benefit favoring osimertinib in terms of DFS was observed irrespective of the use of adjuvant chemotherapy.2 The 24-month DFS rate of 89% was observed regardless of the adjuvant chemotherapy (vs. 49% with placebo, HR=0.16 for adjuvant chemotherapy; vs. 58%, HR=0.23 for no adjuvant chemotherapy).2 Low frequency of dose modifications and treatment discontinuations were also noted from osimertinib, with no new safety signals reported in the ADAURA trial (table 1).2 The most common adverse events (AEs) reported were diarrhea (46%), paronychia (25%), dry skin (23%), pruritus (19%), cough (18%), and stomatitis (18%).2
When considering treatment for local NSCLC cases, it should be noted that the treatment initiation of adjuvant targeted therapies, including osimertinib, depends on the presence of specific molecular mutations such as sensitizing EGFR mutations.3 Asian patients have a higher prevalence of EGFR mutations in exons 18 to exon 22 when compared with Caucasians (30% vs. 7%).5 Therefore, a considerable proportion of local patients can benefit from EGFR-TKIs, which has been demonstrated in the shared local case with EGFR L858R mutated lung adenocarcinoma where adjuvant osimertinib showed its clinical benefits with limited toxicities. The survival benefit of osimertinib, both in study and in practice, in turn highlighted the importance of performing molecular testing on key established predictive biomarkers prior to treatment, which is also recommended by the NCCN guidelines, to ensure that the optimal available treatment is prescribed.3
Moreover, chemotherapy compliance in the post-surgery setting is relatively poor, reflecting in the patient’s refusal of receiving adjuvant chemotherapy in the shared case.6 In the ADAURA study, only 26% of patients with stage IB disease and 76% of patients with stage II to IIIA received adjuvant chemotherapy.2 However, the survival benefit was observed, regardless of the adjuvant chemotherapy, with the same rate (89%) of DFS at 24 months in both the adjuvant osimertinib monotherapy group and the adjuvant osimertinib with chemotherapy combination group.2
Although adjuvant chemotherapy plus osimertinib is still preferred in high-risk patients with visceral pleura or LVI, adjuvant osimertinib monotherapy can be considered in patients in early stages without risk factors of disease recurrence. That said, the patient in this shared case had stage IB lung adenocarcinoma with high-risk features and was still able to achieve disease control with substantial improvements in the quality of life (QoL). “Adjuvant osimertinib was well-tolerated and the patient could work as usual during the treatment course, indicating that osimertinib alone could provide a better QoL having fewer side effects compared with chemotherapy,” highlighted Dr. Tsang. While this patient had mild rashes and neutropenia after receiving osimertinib for 9 months, those events were found to be consistent with the established safety profile in a recent real-world data and meta-analysis of published clinical trials on the use of osimertinib.7,8 Therefore, adjuvant osimertinib monotherapy may be an option for protecting patients with early-stage disease against the chemotherapy induced AEs and disease recurrence. Along with routine and close monitoring on liver function, ejection fraction, ECG, and echocardiogram, osimertinib monotherapy could be a new and desirable option for patients with early-stage resected NSCLC.
Conclusion
Upon detecting EGFR-TKI sensitizing mutations, adjuvant osimertinib should be considered for patients with completely resected NSCLC as early as stage IB, and also for patients with risk factors of disease recurrence. Importantly, the DFS benefit of adjuvant osimertinib monotherapy was supported by both the ADAURA trial and this shared case, which has served to further support the early adoption of osimertinib. In terms of effectively preventing the disease recurrence, osimertinib is a well-tolerated treatment that could provide a better QoL when compared with chemotherapy.

