CONFERENCE UPDATE: ESMO 2023
First-line osimertinib + CT reduces risk of disease progression and death among EGFRm NSCLC patients with CNS metastases: Exploratory analysis of the FLAURA2 study
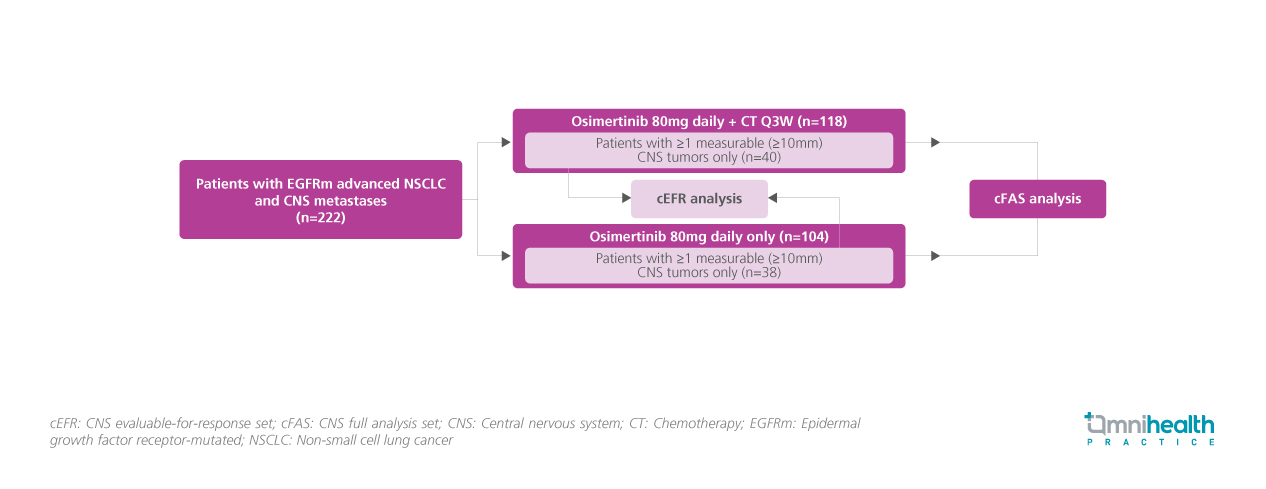
STUDY DESIGN
The phase 2 FLAURA2 study found that first-line combination of osimertinib and chemotherapy (CT) offered significant survival benefits among patients who were diagnosed with epidermal growth factor receptor-mutated (EGFRm) advanced non-small cell lung cancer (NSCLC) compared to first-line osimertinib monotherapy.1 Considering that a majority of patients with EGFRm NSCLC may develop metastasis in other areas of the body such as the central nervous system (CNS), an exploratory analysis based on the patient demographic of the FLAURA2 study was initiated to explore the clinical efficacy of osimertinib combined with CT among EGFRm NSCLC patients who had developed CNS tumors.1
The FLAURA2 study originally enrolled 557 adult patients with pathologically confirmed non-squamous NSCLC with no prior systemic treatments and randomized them into receiving either a daily dose of osimertinib of 80mg + CT once every 3 weeks or osimertinib monotherapy 80 mg daily.1 In this exploratory analysis, patients with CNS metastases who were asymptomatic or considered neurologically stable for ≥2 weeks after definitive treatment (n=222) were selected and categorized into 2 analysis sets, the CNS full analysis set (cFAS) and the CNS evaluable-for-response (cEFR) set.1 The cFAS consisted of patients with ≥1 measurable (≥10mm) and non-measurable CNS tumors at baseline (osimertinib + CT: n=118, osimertinib only: n=104), while the cEFR only consisted of patients with ≥1 measurable (≥10mm) CNS tumors (osimertinib + CT: n=40, osimertinib only: n=38).1
The key exploratory endpoints for this CNS-focused analysis of the FLAURA2 study included CNS progression-free survival (PFS), CNS overall response rate (ORR), CNS duration of response (DoR), and safety in both treatment groups.1
FINDINGS
| Key exploratory endpoints: |
|
|
|
|
|
| Safety: |
|
|
“The results in this exploratory analysis support the addition of chemotherapy to osimertinib as a novel first-line treatment option for patients with EGFRm advanced NSCLC, including those with CNS metastases."
Dr. David Planchard
Department of Cancer Medicine,
Institut Gustave Roussy Villejuif,
France

