CONFERENCE UPDATE: ESMO 2023
Enfortumab vedotin plus pembrolizumab significantly improves outcomes compared to chemotherapy in patients with previously untreated locally advanced metastatic urothelial carcinoma: The EV-302/KEYNOTE-A39 study
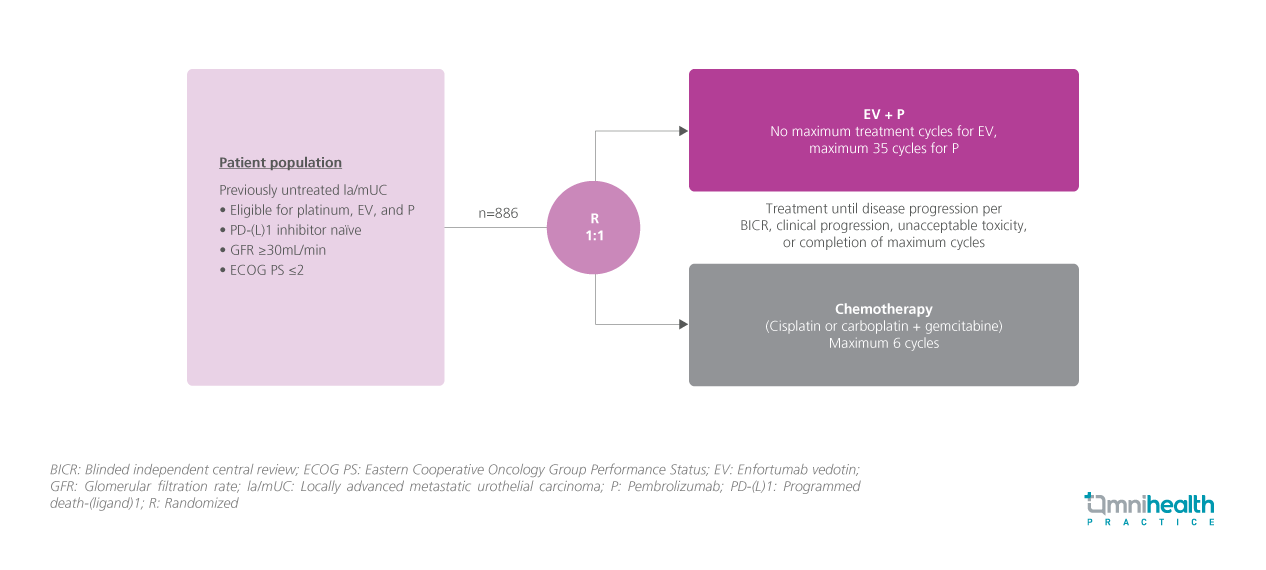
STUDY DESIGN
While platinum-based chemotherapy (CTx) has been the standard first-line (1L) treatment for locally advanced metastatic urothelial carcinoma (la/mUC) for decades, many patients continue to experience disease progression after treatment, resulting in poor prognosis and low 5-year survival rates.1 Combinations with platinum-based CTx with programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have also been suboptimal in improving the survival outcomes of patients with la/mUC.1 Enfortumab vedotin (EV), a Nectin-4-directed antibody-drug conjugate, demonstrated a survival benefit in la/mUC patients who had previously been treated. The EV-302/KEYNOTE-A39 study was conducted to evaluate the efficacy of combining EV with pembrolizumab (P), a PD-1 inhibitor in patients with previously untreated la/mUC against conventional CTx, regardless of cisplatin eligibility and PD-L1 expression status.1
The study recruited 886 patients with previously untreated la/mUC and randomized them 1:1 to either receive EV+P for a maximum of 35 cycles (n=440) or CTx (cisplatin or carboplatin + gemcitabine) for a maximum of 6 cycles (n=433).1 Patients were treated until disease progression, unacceptable toxicity, or completion of maximum cycles.1 Key demographic and baseline disease characteristics were balanced between treatment arms and representative of the 1L la/mUC population.1
The primary endpoints were progression-free survival (PFS) by blinded independent central review (BICR) and overall survival (OS). Secondary endpoints included overall response rate (ORR) per RECIST v1.1 by BICR and investigator assessment, and safety.1 In the subgroup analysis, results were stratified by age, sex, ECOG PS, primary disease site of origin, cisplatin eligibility, level of PD-L1 expression, and presence of liver metastases.
| Primary endpoints: |
|
|
|
|
| Secondary endpoints: |
|
|
| Safety: |
|
|
|
|
“EV-302/KEYNOTE-A39 is the first time that platinum-based chemotherapy has been surpassed in OS in patients with previously untreated la/mUC. These results support EV+P as a potential new standard of care for 1L la/mUC.”
Professor Thomas Powles
Barts Cancer Centre,
London, United Kingdom

