CONFERENCE UPDATE: ESMO 2023
Survival benefits of neoadjuvant nivolumab chemotherapy combination in patients with resectable NSCLC regardless of PD-L1 expression maintained in 3-year exploratory analysis from the CheckMate 816 trial
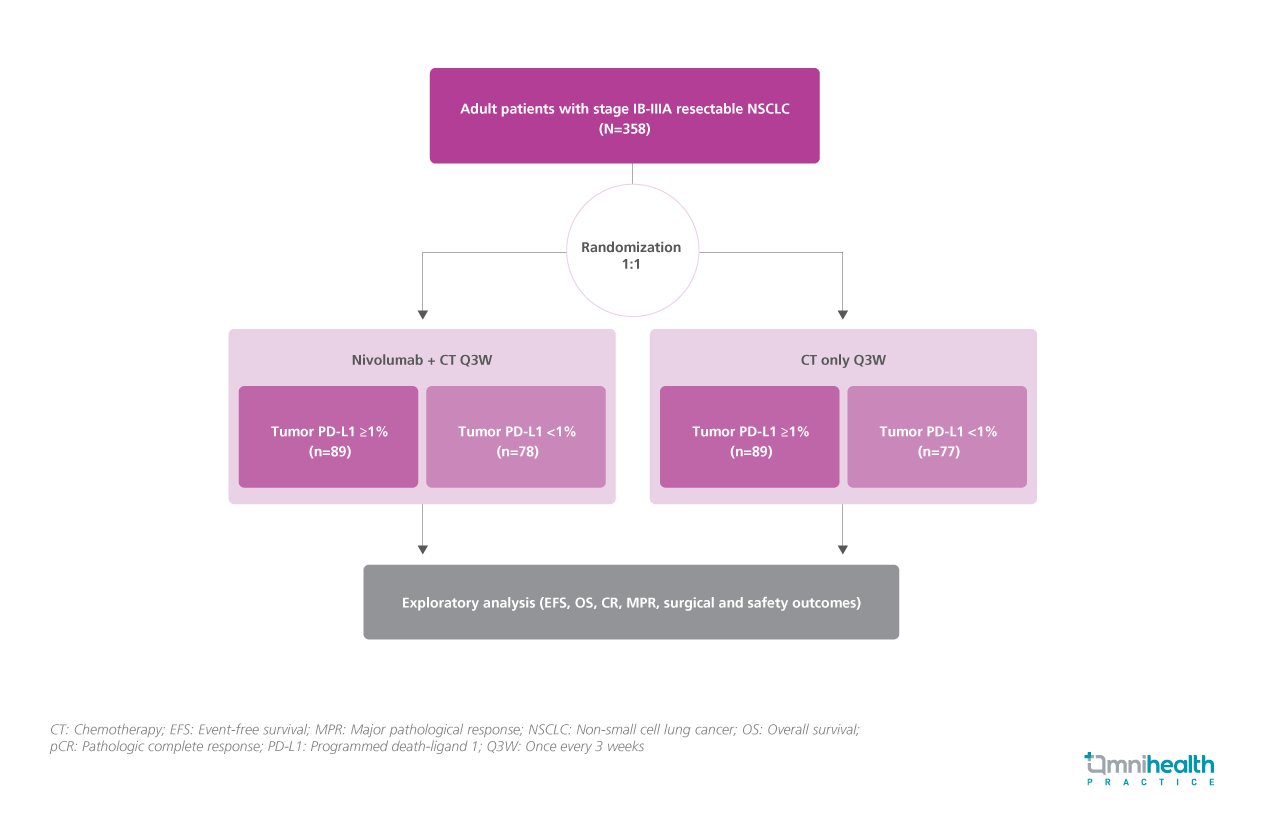
STUDY DESIGN
Results from the phase 3 CheckMate 816 study had found that neoadjuvant nivolumab plus chemotherapy (CTx) induced clinically significant and sustained benefits in the event-free survival (EFS) and overall survival (OS) of patients with resectable non-small cell lung cancer (NSCLC) compared to CTx-only.1 An exploratory subgroup analysis at 3 years was conducted to investigate the efficacy and safety outcomes of this combined neoadjuvant treatment among NSCLC patients by tumor programmed death-ligand 1 (PD-L1) expression (≥1% or <1%).1
During the phase 3 CheckMate 816 Study, adult patients with stage IB–III resectable NSCLC were randomized 1:1 to receive 360mg of nivolumab plus CTx once every 3 weeks or CTx only once every 3 weeks for 3 cycles.1 Both treatment groups consisted of similar proportions of patients with a tumor PD-L1 expression of ≥1% (nivolumab + CTx=89, CTx=89) and <1% (nivolumab + CTx=78, CTx=77).1
The endpoints of this exploratory analysis included event-free survival (EFS), overall survival (OS), pathologic complete response (pCR), major pathological response (MPR), surgical outcomes, and safety in patients with tumor PD-L1 ≥ 1% or < 1%.1 The minimum and median follow-ups were 32.9 months and 41.1 months, respectively.
| Exploratory endpoints: |
|
|
|
|
|
|
|
|
|
| Safety: |
|
“Results of this exploratory analysis reinforce the role of nivolumab plus chemotherapy as a standard neoadjuvant treatment for patients with resectable NSCLC, regardless of tumor PD-L1 expression.”
Dr. Mariano Provencio Pulla
Medical Oncology Department,
Puerta de Hierro University Hospital
Madrid, Spain

