CONFERENCE UPDATE: ESMO 2025
Lenvatinib + pembrolizumab delivers sustained efficacy in aRCC regardless of baseline bone metastases: Final analysis from CLEAR
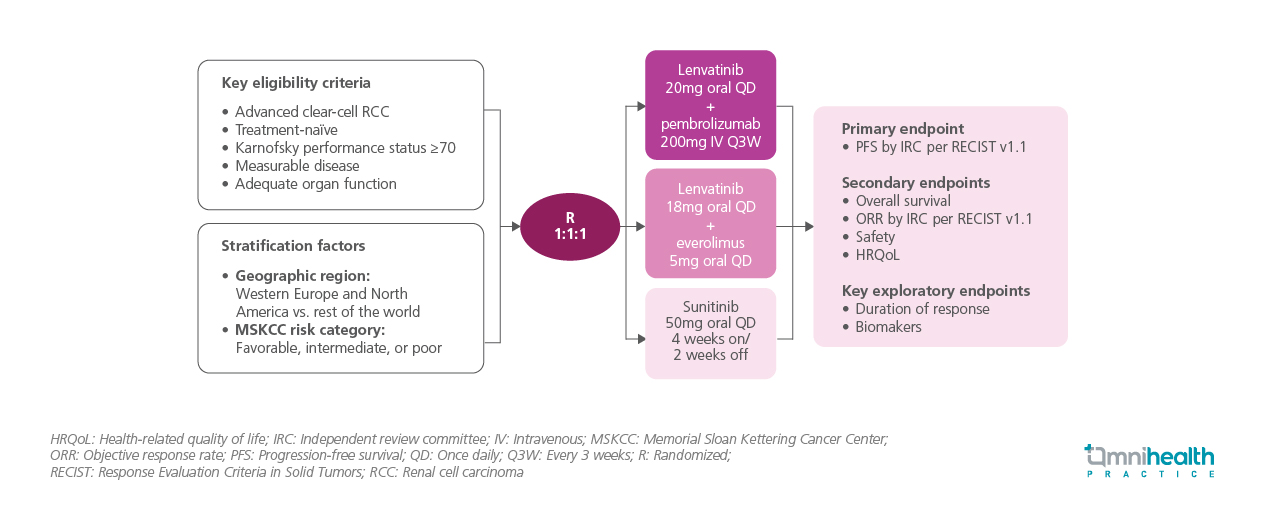
STUDY DESIGN
Bone metastases occur in approximately one-third of patients with advanced renal cell carcinoma (aRCC) and are associated with poor prognosis. This is partly due to RCC-driven immune dysregulation and the release of factors such as fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) that promote osteoclast activation and bone resorption.1 Dysregulation of the FGF or FGF receptor (FGFR) axis further contributes to bone metastases development.1 Lenvatinib is an oral tyrosine kinase inhibitor (TKI) that targets several growth factor receptors, including VEGFR1-3, FGFR1-4, PDGFRα, CD117 (KIT), and the RET proto-oncogene.1 When combined with pembrolizumab, it has shown significant improvements in progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) compared to sunitinib in first-line advanced RCC, with benefits sustained after 4 years of follow-up.1 The captioned analysis presents additional outcomes in patients with and without bone metastases after approximately 4 years of follow-up.1
The CLEAR trial is a global, phase 3, randomized study involving 1,069 patients with treatment-naïve advanced clear cell RCC.1 Eligible patients had measurable disease, adequate organ function, and a Karnofsky performance status (KPS) ≥70.1 Patients were stratified by geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) risk groups before being randomized in a 1:1:1 ratio to receive oral lenvatinib 20mg once daily (QD) + intravenous (IV) pembrolizumab 200mg every 3 weeks, lenvatinib + everolimus, or sunitinib 50mg QD on a 4-weeks-on/2-weeks-off schedule.1 Tumor responses were assessed via independent imaging review and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score changes were evaluated 6 months after treatment initiation.1 Baseline characteristics were generally balanced across treatment arms for patients both with and without bone metastases.1 Notably, few patients with bone metastases had received prior bone-targeted therapies, which were more commonly used in the sunitinib arm than in the lenvatinib + pembrolizumab arm.1
The primary endpoint of the trial was independent review committee (IRC)-assessed PFS.1 Key secondary endpoints included OS, ORR, health-related quality of life (HRQoL), and safety.1 Key exploratory endpoints were duration of response (DoR) and biomarkers analyses.1
FINDINGS
| Primary endpoint: |
|
| Secondary endpoints: |
|
| Key exploratory endpoints: |
|
“OS, PFS, ORR, DoR and IMDC risk improvement continue to support lenvatinib + pembrolizumab as a standard-of-care treatment for patients with aRCC, regardless of the baseline bone metastases status.”
Professor Camillo Porta
University of Bari Aldo Moro & Policlinico Consorziale,
Bari, Italy

