CONFERENCE UPDATE: ASN 2023
Identifying the mediators of dapagliflozin-induced renal benefits in patients with CKD with or without T2DM: Post-hoc analysis of the DAPD-CKD trial
Study Design
Dapagliflozin, a sodium-glucose cotransporter 2 inhibitor (SGLT2i), has been shown to induce renal, cardiovascular (CV), and survival benefits in patients with chronic kidney disease (CKD), regardless of the presence of type 2 diabetes mellitus (T2DM) in the DAPA-CKD trial.1 Separate mediation analyses performed using clinical data from patients with T2DM revealed that the renal benefits of SGLT2is can be attributed to several key mediators such as hemoglobin, urinary albumin-to-creatinine ratio (UACR), and serum urate.1 Nonetheless, whether these mediators are involved in facilitating the observed kidney-protective benefits of dapagliflozin in patients with CKD with or without T2DM remained unclear.1
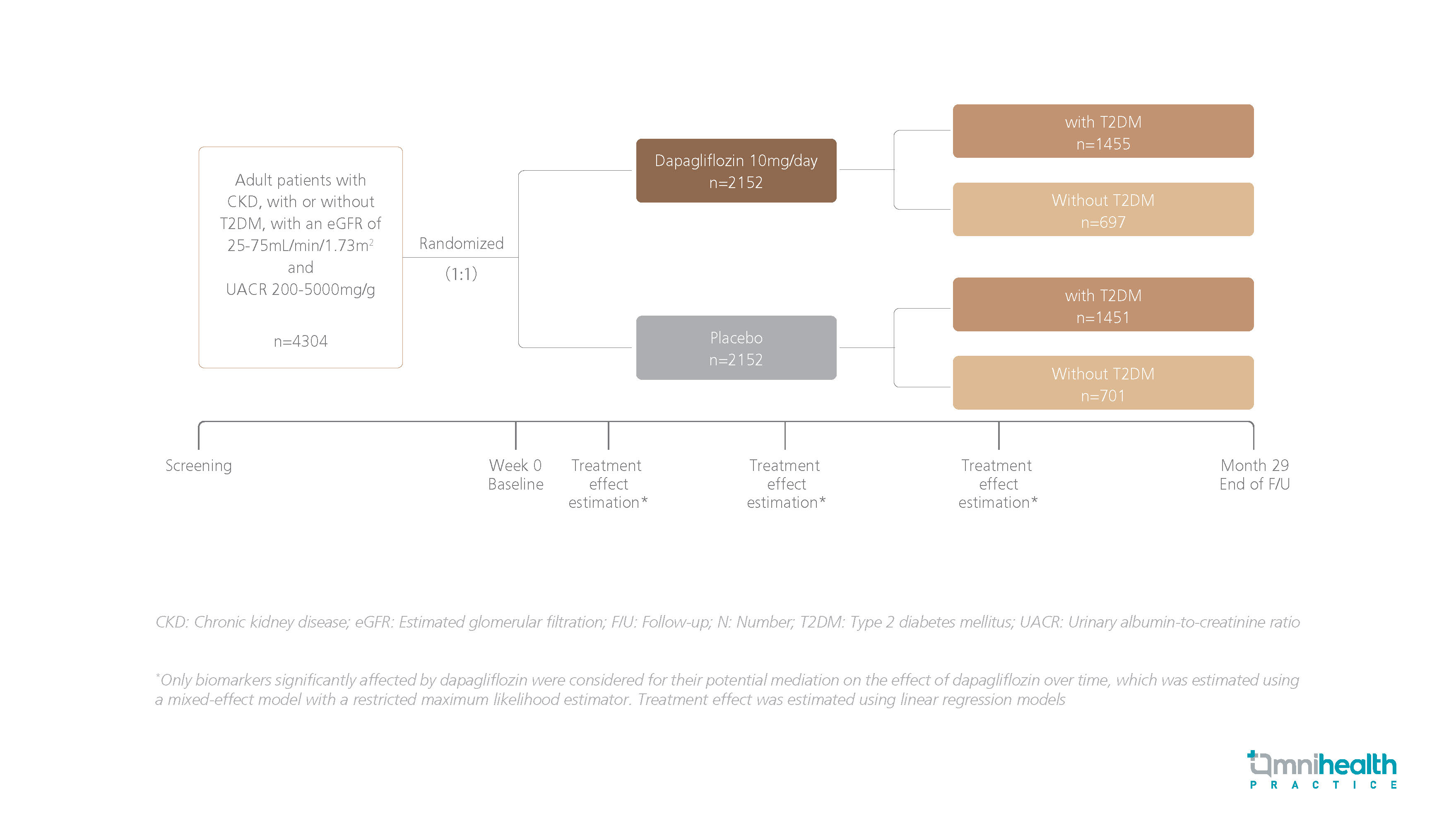
A post-hoc analysis of the DAPA-CKD trial was conducted to examine the potential mediators of the observed kidney-protective effects of dapagliflozin in patients with CKD, with or without T2DM.1 A total of 4,304 adult participants with CKD, with or without T2DM, with an estimated glomerular filtration rate (eGFR) of 25mL/min/1.73m2-75mL/min/1.73m2 and UACR of 200mg/g-5000mg/g were randomized to receive dapagliflozin 10mg daily (n=2,152) or placebo (n=2,152) with a median follow-up of 2.4 years.1 Biomarkers including UACR, hematocrit, blood pressure (BP), body weight, heart rate, HbA1c, serum potassium, and serum sodium levels were examined throughout the study.1
The primary endpoint was a composite of ≥50% sustained eGFR decline, end-stage kidney disease (ESKD), or CV- or renal-related deaths.1 The prespecified kidney outcome encompassed outcomes of the primary endpoint, excluding CV death.1
|
Primary outcome: |
|
|
Kidney outcome: |
|

