CASE REVIEW
Dapagliflozin adds to the treatment options against IgA nephropathy: A local case sharing
Immunoglobulin A nephropathy (IgAN) is the most common type of glomerulonephritis (GN), with a higher prevalence in Asia compared with the Western world.1-3 The current mainstay of IgAN treatment is mainly optimized supportive care.1 Despite the use of renin-angiotensin-aldosterone system (RAAS) inhibitors and immunosuppressants, many IgAN patients still progress to kidney failure.4 Recently, the DAPA-CKD trial has unprecedentedly demonstrated the renal-protective benefits of dapagliflozin among IgAN patients with or without type 2 diabetes (T2D), offering an effective alternative to IgAN patients for improved renal outcomes.4 In an interview with Omnihealth Practice, Dr. Chan, Ting-Pong Anthony adopted this novel option in his practice and shared a clinical case to demonstrate the benefits of dapagliflozin in a young patient whose IgAN was uncontrolled with conventional medications.
Background
IgAN belongs to the most common type of primary GN and is most prevalent in Asia, accounting for 37%-58% of biopsy-proven primary GN in China.1,3,5 The disease is characterized on renal biopsy by the predominant deposition of galactose-deficient-immunoglobulin A (IgA) -containing immune complexes in the glomerular mesangium, leading to glomerulitis and fibrosis of the kidney.4,6 About 15%-40% of IgAN patients will experience a gradual decline of renal function and eventually progress to end-stage renal disease within 20 years of diagnosis.5 In other words, IgAN is associated with significantly increased morbidity and mortality risks among patients.5
Principles and current IgAN management
“While current management approach remains supportive, the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 guideline for glomerular disease management currently recommends that IgAN patients should be preferably managed with lifestyle changes and maximally tolerated doses of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) for blood pressure control and reduction in proteinuria. The target blood pressure should be maintained at systolic blood pressure (SBP) ≤130mmHg and diastolic blood pressure (DBP) ≤90mmHg. The presence of proteinuria of ≥1g increases the risk of progression to end-stage kidney disease. Patients with proteinuria of 0.8-1g/day are still at risk, so the target level should preferably be maintained at <0.75g/day. “Lifestyle modifications shall include smoking cessation, limitation of daily sodium intake, normalization of weight, and undertaking regular physical activities,” Dr. Chan advised.
In fact, some patients might not be responsive to the first-line treatments. “Immunosuppressants are often needed when patients fail to achieve the target level of proteinuria after 6 months of conservative ACEi/ARB therapies,” Dr. Chan said. “Alternatively, mycophenolate mofetil (MMF) is a viable treatment option and is recommended as a corticosteroid-sparing therapy for Chinese IgAN patients,” he suggested.
Limitations of conventional therapies and unmet needs in IgAN
RAAS blockade (ie., ACEis/ARBs) remains the mainstay of IgAN management, and dose optimization is needed to achieve the target level of proteinuria.7 “Nevertheless, when high doses of ACEis/ARBs are given, dizziness, fatigue or hypotension can occur and is common in IgAN patients. Also, despite high patient adherence to potassium restriction, hyperkalemia could still happen in some patients with dose escalation of the RAAS inhibitors,” Dr. Chan said. On top of ACEis/ARBs, the corticosteroid therapy is often offered to patients who do not respond to supportive measures.4 “However, the use of corticosteroids has been associated with increased risks of infections, metabolic complications, such as steroid-induced diabetes, weight gain and even psychiatric problems. Other immunosuppressive agents, such as MMF, are also associated with deranged liver function and increased risk of infections,” he stressed. An additional treatment option is therefore warranted for IgAN patients to preserve the renal function with high efficacy and good safety.
The emerging role of dapagliflozin in IgAN
Dapagliflozin is a sodium-glucose cotransporter-2 inhibitor (SGLT2i), which was initially developed for managing T2D and was subsequently approved for the treatment of heart failure with reduced ejection fraction (HFrEF).8 In early cardiovascular (CV) outcome trials, considerable renal benefits were observed with SGLT2is in T2D patients.4
DAPA-CKD, a dedicated renal outcome trial, was therefore designed for assessing the renal benefits of dapagliflozin in chronic kidney disease (CKD) patients with or without T2D (n=4,304).4,9 After a median follow-up of 2.4 years, the results showed that dapagliflozin significantly reduced the primary composite outcome of a sustained ≥50% decline in estimated glomerular filtration rate (eGFR), end-stage kidney disease, or death from renal or CV causes among CKD patients, regardless of the T2D status (HR=0.61; 95% CI: 0.51-0.72; p<0.001).9 Furthermore, the placebo-like safety profile of dapagliflozin in the trial has reassured clinicians, supporting its extensive utilization in the CKD management.9
More recently, a prespecified subgroup analysis of the DAPA-CKD trial has demonstrated the remarkable and consistent renal benefits of dapagliflozin among IgAN patients (n=137 out of 4,304).4 After a median follow-up of 2.1 years, dapagliflozin was shown to significantly reduce the risk of primary composite outcomes among IgAN patients by 71% (HR=0.29; 95% CI: 0.12-0.73; p=0.005), with an absolute risk difference of -10.7% (95% CI: -17.6% to -3.7%) vs. placebo (figure 1).4
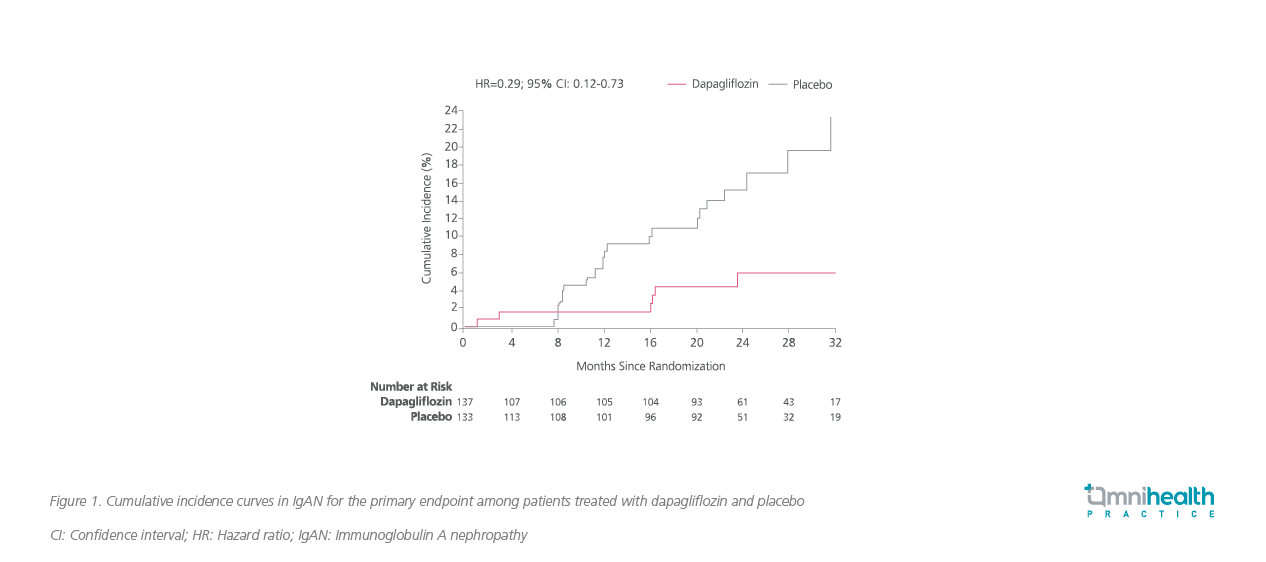
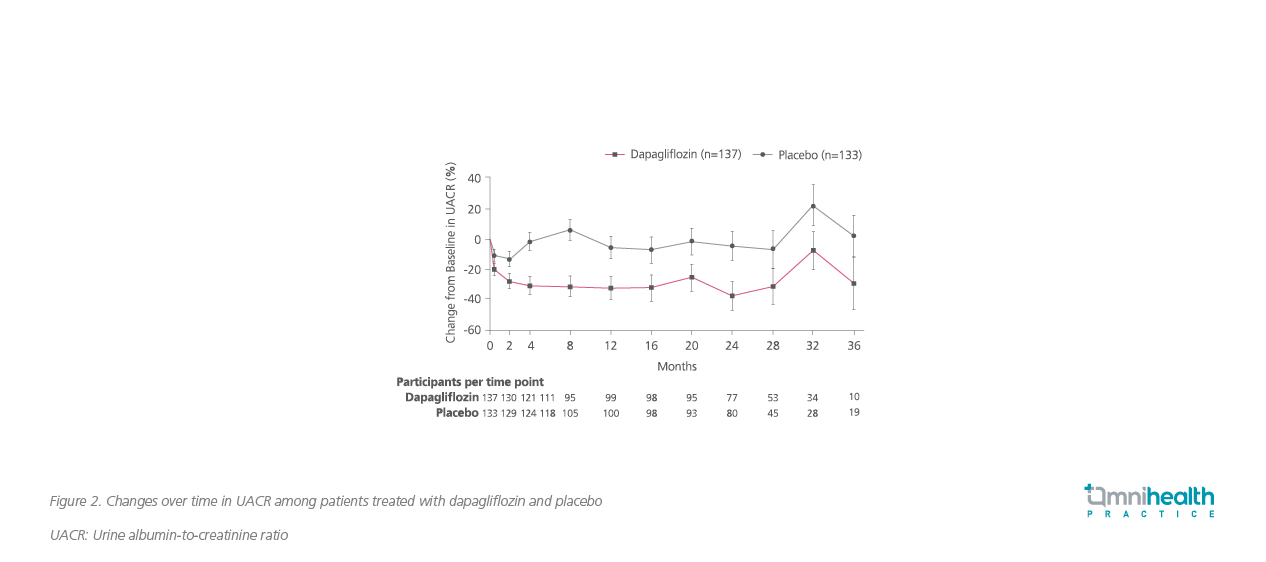
Significant reductions in albuminuria and blood pressure were also observed among IgAN patients treated with dapagliflozin. Dapagliflozin significantly reduced urine albumin-to-creatinine ratio (UACR) by 26% (95% CI: -37% to -14%; p<0.001) vs. placebo (figure 2).4 Dapagliflozin also significantly reduced SBP and DBP by 3.5mmHg (95% CI: 5.7-1.3; p=0.002) and 2.2mmHg (95% CI: 3.7-0.8; p=0.003) vs. placebo, respectively.4
Overall, dapagliflozin was generally well tolerated among IgAN patients. The rate of adverse events (AEs) leading to treatment discontinuation was comparable between dapagliflozin and placebo (4.4% and 5.3%, respectively). None of the participants developed major hypoglycemia or diabetic ketoacidosis (DKA).4
Local case sharing
A 33-year-old female with previous gross hematuria during upper respiratory tract infection and fever at the age of 15. She was referred to the renal clinic in 2012 due to persistent microscopic hematuria and proteinuria. A renal biopsy confirmed the diagnosis of IgA nephropathy.
She has been receiving various treatments over the years. Her first prescribed medication was ramipril, but she could not tolerate it due to her persistent dry cough. She then switched to olmesartan 20mg once daily, but the response was poor: Her 24-hour urine protein was subsequently increased to 2.75g per day. In light of this, MMF 500mg was added in conjunction with the RAAS inhibitor in 2013, stabilizing her proteinuria and renal function with 24-hour urine protein staying between 0.75g to 1.8g per day and creatinine between 85-105μmol/L.
However, in June 2021, the patient's condition suddenly deteriorated, with the 24 hours urine protein level jumping from 0.8g/day to 4.2g/day despite the existing ARB and MMF treatment. Her previous nephrologist increased the dose of MMF and added oral corticosteroids immediately to address the worsening condition. Unfortunately, the addition of immunosuppressive therapies to the background ARB therapy failed to significantly reduce the 24-hour urine protein level, which was just down to 3.71g/day from 4.2g/day.
Given the potentially severe adverse effects of increasing the dosages of MMF and oral corticosteroids, the preferred option was to further increase the dose of olmesartan from 20mg to 30mg daily. Nevertheless, the patient could not tolerate the high dose of olmesartan and reported severe dizziness and fatigue, which seriously affected her daily functioning. Also, her SBP dropped significantly to around 85-90mmHg. The patient then resumed the maximum tolerated dose of olmesartan 20mg daily.
In December 2021, dapagliflozin therapy was started after discussing its risks and benefits with the patient. Her 24-hour urine protein level showed a rapid and significant reduction from 3.71g/day to 1.2g/day after 3 months of initiating dapagliflozin. The dapagliflozin treatment stabilized her worsening eGFR, and a marked and sustained improvement in proteinuria was observed. In view of the improved proteinuria, prednisolone was weaned off in April 2022 to prevent steroid complications. She was followed up in September 2022, and her 24-hour urine protein was down to 0.71g per day (figure 3). Her creatinine level was 122mmol/L, which is stable.
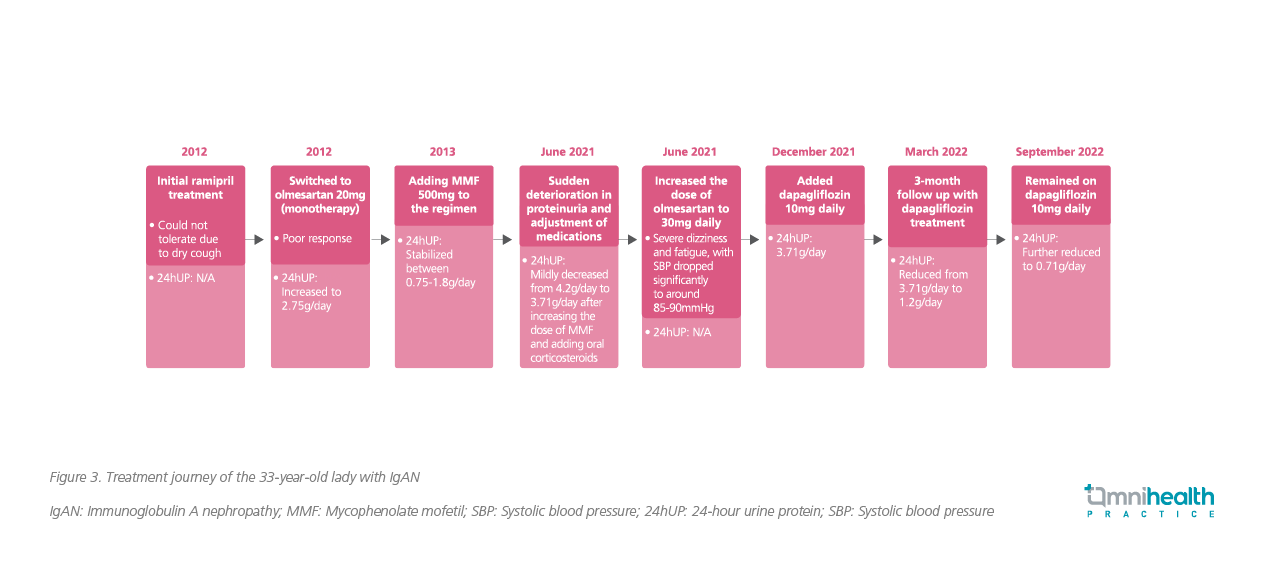
The patient showed good compliance with dapagliflozin and now remains on the treatment with dapagliflozin 10mg daily, olmesartan 20mg daily, prednisolone 2.5mg on alternate days, and MMF 500mg twice daily. Overall, she tolerated the regimen well and did not report any AEs.
Discussion
“It is now known that the reno-protective effect of dapagliflozin is due to its vasoconstriction effect on the afferent arteriole which reduces glomerular hyperfiltration, thus protecting the kidney from further injury and reducing the progression of CKD,” Dr. Chan said. "Notably, dapagliflozin and RAAS inhibitors synergize and change the renal hemodynamics, thus ameliorating proteinuria; in combination with corticosteroids and MMF, dapagliflozin downregulates the inflammatory process of IgAN and reduces the scarring and intracellular matrix deposition.“
Regarding the clinical use of dapagliflozin, Dr. Chan reminded that since an initial dip in eGFR with dapagliflozin could happen, which is normal owing to its mechanism of action, patients should be followed up within 4-6 weeks upon initiation of the SGLT2i for monitoring the renal function, and thereafter, every 2 months to see if the renal function is stable or if there is any improvement in proteinuria. According to Dr. Chan’s clinical experiences, dapagliflozin was well tolerated in most of his patients. However, he noted that the long-term data for SGLT2i by the IgAN management is still lacking. Results of a longer trial is therefore needed to demonstrate the long-term benefits among IgAN patients.
Conclusion
In summary, the use of conventional treatments, including RAAS inhibitors and immunosuppressants, could be insufficient to slow down IgAN progression and achieve the target level of proteinuria. In addition, dose optimization (i.e., dose escalation) is also limited by serious side effects. “Based on the clinical experiences and the positive results from the prespecified subgroup analysis of the DAPA-CKD trial in IgAN patients, it is concluded that dapagliflozin has a potential role in reducing albuminuria and the progression of CKD in the local practice, offering a new option to the IgAN management”, Dr. Chan said. Clinicians are advised to be more open to adopting dapagliflozin as a novel adjunct therapy to ACEi/ARB therapies for achieving better treatment outcomes in IgAN. Further dedicated IgAN studies with longer follow-up period will also be able to provide more insights on SGLT2 inhibitors in IgAN patients.
This is an independent editorial article, published and distributed through unrestricted educational support from AstraZeneca Hong Kong Limited, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Floege J, et al. Current treatment of IgA nephropathy. Semin Immunopathol. 2021; 43(5):717-728.
- Schena FP, et al. Epidemiology of IgA nephropathy: A global perspective. Semin Nephrol. 2018;38(5):435-442.
- Zhu L, et al. The genetics of IgA nephropathy: An overview from China. Kidney Dis (Basel). 2015; 1(1):27-32.
- Wheeler DC, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215-224.
- Xie J, et al. Predicting progression of IgA Nephropathy: New clinical progression risk score. Plos One. 2012;7(6):e38904.
- Rajasekaran A, et al. IgA nephropathy: An interesting autoimmune kidney disease. Am J Med Sci. 2021;361(2):176-194.
- Rovin BH, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753-779.
- Prescribing information of FORXIGA. AstraZeneca Hong Kong.
- Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446.
Dapagliflozin reduces recurrent cardiovascular events in patients with HFrEF
According to the DAPA-HF trial, the sodium-glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin, added to standard therapy, reduced the risk of worsening heart failure (HF) and cardiovascular (CV) death in HF patients with reduced ejection fraction (HFrEF). However, conventional time-to-first event (TTFE)

