MEETING HIGHLIGHT
Breaking the vicious cycle of cardiac, renal and metabolic risks in patients at risk of chronic kidney disease
Despite being associated with significant cardiorenal outcomes, many diabetic patients remain undiagnosed of chronic kidney diseases (CKD) or heart failure (HF) even when their diseases are at an advanced stage.1-3 In the recent Advances in Medicine 2021 held by the Department of Medicine and Therapeutics of The Chinese University of Hong Kong, Professor Carol Pollock from the Kolling Institute of Royal North Shore Hospital and the University of Sydney, synthesized the clinical trial data of managing renal and cardiovascular complications with sodium glucose co-transporter-2 inhibitors (SGLT2i) in patients with or without type 2 diabetes mellitus (T2DM) and recommended the early detection and intervention of cardiorenal outcomes for better disease management.
Unmet needs in managing the intricately linked renal and cardiovascular outcomes
T2DM affects 462 million people worldwide, and 40% of diabetic patients will develop diabetic kidney disease that leads to end-stage renal disease (ESRD).1,4 Concurrently, diabetic patients are twice as likely to die from cardiovascular causes than non-diabetic patients.1 Although the global prevalence of CKD was 698 million in 2017 which far exceeded that of HF at 64 million, Prof. Pollock remarked that renal and cardiovascular dysfunctions are interrelated and can lead to a vicious cycle of cardiac, renal and metabolic risks that perpetuates the combined disorder of the two organ systems.4,5 Indeed, patients who developed combined outcomes have the highest risks of adverse cardiovascular outcome and all-cause mortality (HR=3.91 and 3.14, respectively).6
While early intervention can improve cardiorenal outcomes, 28% of T2DM patients aged ≥60 years were found to have previously unknown HF only during additional cardiac screening.2,7 Similarly, 47% of T2DM patients were unaware of their CKD status even during advanced stage 4 disease.3 In fact, only 43% of patients without a CKD diagnosis but confirmed diabetes and hypertension had urine albumin testing, resulting in a third of incident ESRD patients receiving little to no pre-ESRD nephrology care.8 Subsequent to this low CKD recognition rate, Prof. Pollock commented that, “Very worryingly, only 21% of patients with a known diagnosis of CKD were prescribed with renin-angiotensin system (RAS) inhibitors.”9 To prevent new onset or worsening of renal function, Prof. Pollock emphasized the pressing need to implement routine screening for earlier treatment initiation.
Current options have limited capacity in preventing new onset or worsening of renal function
Although RAS blockade remains the standard-of-care for diabetic nephropathy, Prof. Pollock noted that the global age-standardized mortality rate for CKD has not improved in the past decade.10 Notably, the renal replacement therapy (RRT) burden in Asia is expected to more than double from 0.968 million in 2010 to 2.162 million in 2030, urging the need to improve the currently limited treatment to CKD.11
While dual RAS blockade with angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) can further inhibit the RAS system, this combination had worsened the composite outcome of dialysis, doubling of serum creatinine and death (HR=1.09; 95% CI: 1.01-1.18; p=0.037).12 Furthermore, this combination therapy offered no renal benefit but was associated with an excess risk of hyperkalemia and acute kidney injury (AKI; both p<0.001).13 Alternatively, bardoxolone methyl, an antioxidative inflammatory modulator, was explored but failed to reduce the risk of ESRD and had significantly increased hospitalization or death from HF (HR=1.83; 95% CI: 1.32–2.55; p<0.001).14 “There is some data about treatment to prevent new onset or worsening of renal function in patients with diabetic kidney disease, but the data is quite limited,” stated Prof. Pollock when evaluating the contradicting data to support the cardiorenal benefits of RAS inhibitors.
Dapagliflozin, the SGLT2i supported with the most evidence in treating diabetic kidney disease
To address the limitations of RAS blockage, Prof. Pollock underlined SGLT2i as promising agents that improved albuminuria and creatininebased kidney outcomes.15 In a meta-analysis of 4 major SGLT2i studies, namely EMPA-REG OUTCOME, CANVAS, CREDENCE, and DECLARE-TIMI 58, SGLT2i substantially reduced the risk of dialysis, transplantation or death due to kidney disease (RR=0.67; 95% CI: 0.52-0.86; p=0.0019).15 Moreover, SGLT2i significantly reduced the risk of ESRD (RR=0.65; 95% CI: 0.53–0.81; p<0.0001) and AKI (RR=0.75; 95% CI: 0.66-0.85; p<0.0001) with consistent benefit across baseline eGFR subgroups.15
When considering study representativeness, Prof. Pollock noted that the DECLARE-TIMI 58 trial had the highest applicability to the real-world based on the patient population and study outcomes.16 In this study (n=17,160), dapagliflozin significantly reduced the risk of cardiorenal composite outcome by 24% among T2DM patients with a relatively good mean eGFR of 85.2mL/min/1.73m2 (HR=0.76; 95% CI: 0.67-0.87; p<0.0001).17 In terms of renal-specific outcome, an even greater risk reduction of 47% was observed (HR=0.53; 95% CI: 0.43-0.66; p<0.0001).18 Impressively, dapagliflozin was associated with a 46% reduction in sustained eGFR decline by at least 40% to <60ml/min/1.73m2 (HR=0.54; 95% CI: 0.43-0.67; p<0.0001) and a significantly lower risk of ESRD or renal death (HR=0.41; 95% CI: 0.20-0.82; p=0.012) even when the majority of T2DM patients (81.3%) were already receiving RAS inhibitors, supporting the benefit of dapagliflozin to be cumulative to RAS blockade.17,18 Regardless of T2DM duration, dapagliflozin consistently reduced the risk of cardiovascular death or hospitalization for HF among those with ≤5 years (HR=0.79; 95% CI: 0.58-1.06) or >20 years of diabetes (HR=0.75; 95% CI: 0.55-1.03; pinteraction=0.76).19 In particular, dapagliflozin significantly reduced the risk of major cardiovascular outcome (MACE) among patients with >20 years of diabetes (HR=0.67; 95% CI: 0.52-0.86) that was heavily driven by the reduction of myocardial infarction (MI; HR=0.66; 95% CI: 0.47-0.92) and ischaemic stroke (HR=0.61; 95% CI: 0.38-1.00).19 “Dapagliflozin has outcome studies in CKD, cardiovascular and HF trials for a more comprehensive totality of data,” commented Prof. Pollock.
The renal benefit of dapagliflozin extends beyond diabetes status
To evaluate the renal-specific benefit of dapagliflozin in patients without T2DM, the randomized, double-blind, placebo-controlled, multicenter DAPA-CKD study (n=4,304) recruited patients with or without T2DM to assess the primary composite outcome of sustained ≥50% eGFR decline, ESRD, renal or cardiovascular death.20 In this study population, 49% had a urinary albumin-to creatinine ratio (UACR) of >1,000, 98% were already receiving standard renal-protective therapies and 68% had T2DM.20
After a median follow-up of 2.4 years, dapagliflozin significantly slowed the progression of kidney disease with a 39% relative risk reduction (RRR) in the primary composite outcome when compared to placebo (HR=0.61; 95% CI: 0.51-0.72; p=0.000000028; Figure 1).20 Moreover, dapagliflozin reduced the composite of cardiovascular death or hospitalization for HF (HR=0.71; 95% CI: 0.55-0.92; p=0.0089) and all-cause mortality (HR=0.69; 95% CI: 0.53-0.88; p=0.0035).20 Prof. Pollock remarked that there was an even greater reduction in RRR of 44% in the renal-specific outcome, highlighting the cardiorenal benefit of dapagliflozin to be independent of its glucose-lowering effect which was consistent across patients with (HR=0.64; 95% CI: 0.52-0.79) or without T2DM (HR=0.50; 95% CI: 0.35-0.72).20
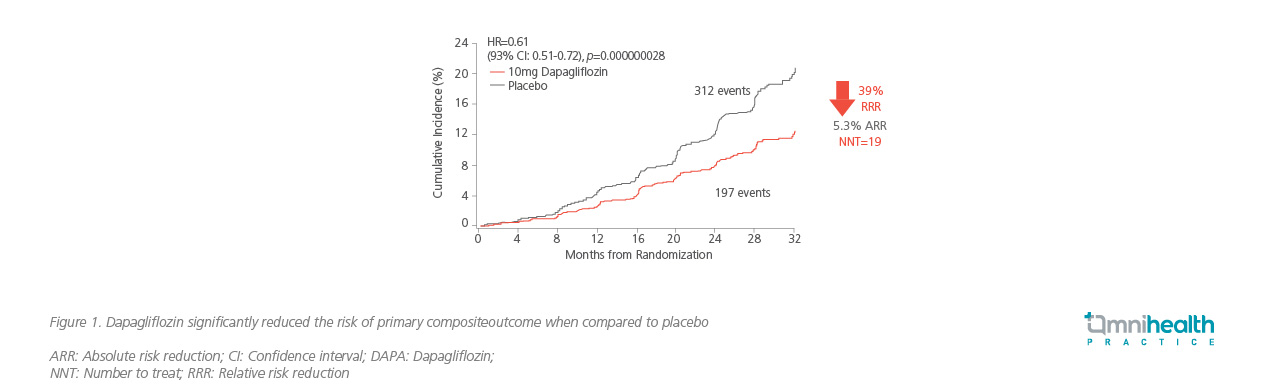
Compared to the CANVAS and CREDANCE studies, Prof. Pollock noted that the DAPA-CKD study was the only trial that demonstrated a renal benefit in patients with stage 4 CKD (HR=0.73; 95% CI: 0.53-1.02).21 Notably, this cardiorenal benefit of dapagliflozin was consistent regardless of eGFR when accounting for the primary composite outcome (p=0.22), ESRD (p=0.64) or kidney or cardiovascular death (p=0.74), stressing the benefit of initiating dapagliflozin even in patients with an eGFR of <30mL/min/1.73m2.21 In patients with immunoglobulin A (IgA) nephropathy, dapagliflozin in addition to ACEi/ARB also substantially reduced the risk of CKD progression (HR=0.29; 95% CI: 0.12-0.73), emphasizing the broad applicability of dapagliflozin in the real-world.22
Based on the DAPA-CKD study and its pre-specified analyses, the United States Food and Drug Administration updated the label of dapagliflozin on April 30, 2021 to include the risk reduction of kidney function decline, kidney failure, cardiovascular death, and hospitalization for HF in adults with CKD who are at risk of disease progression.23 With dapagliflozin demonstrating clear cardiorenal benefits, Prof. Pollock summarized that, “Although SGLT2i were originally introduced for glycemic control, their renal protective benefits are completely independent of whether or not people have diabetes.”
Dapagliflozin consistently improves cardiovascular and all-cause mortality outcomes
In addition to renal-specific benefits, dapagliflozin also demonstrated cardioprotective benefit in the DAPA-HF study by significantly reducing the primary outcome of worsening HF or cardiovascular diseases by 26% when compared to placebo (HR=0.74; 95% CI: 0.65-0.85; p<0.001) regardless of the diabetes status or baseline eGFR.24,25 Compared to the EMPEROR-REDUCED study, dapagliflozin significantly reduced the all-cause mortality (HR=0.83; 95% CI: 0.71-0.97), cardiovascular death (HR=0.82; 95% CI: 0.69-0.98) and first hospitalization for HF (HR=0.70; 95% CI: 0.59-0.83), versus empagliflozin only showing significant benefit in first hospitalization for HF (HR=0.69; 95% CI: 0.59-0.81) but not in all-cause mortality (HR=0.92; 95% CI: 0.77-1.10) or CV death (HR=0.92; 95% CI: 0.75-1.12).25 In patients aged >55, dapagliflozin continued to show significant cardiovascular benefit including those aged ≥75 years (HR=0.68; 95% CI: 0.53-0.88), versus empagliflozin only showing a beneficial trend (HR=0.86;0.67-1.10) when treating this elderly population.25 “In patients aged over 75 years, the DAPA-HF study has demonstrated better results than the EMPEROR-REDUCED study,” reiterated Prof. Pollock. Interestingly, when considering all-cause mortality specifically for patients with CKD, Prof. Pollock noted that the survival benefit of dapagliflozin (HR=0.69; 95% CI: 0.53-0.88; p=0.003) was mainly driven by the improvements in non-cardiovascular deaths (HR=0.54; 95% CI: 0.36-0.82).26 Particularly, dapagliflozin consistently reduced the all-cause mortality of patients with (HR=0.74; 95% CI: 0.56-0.98) or without diabetes (HR=0.52; 95% CI: 0.29-0.93; Figure 2) with significant improvement in HF but not in sudden deaths, acute MI or stroke.26
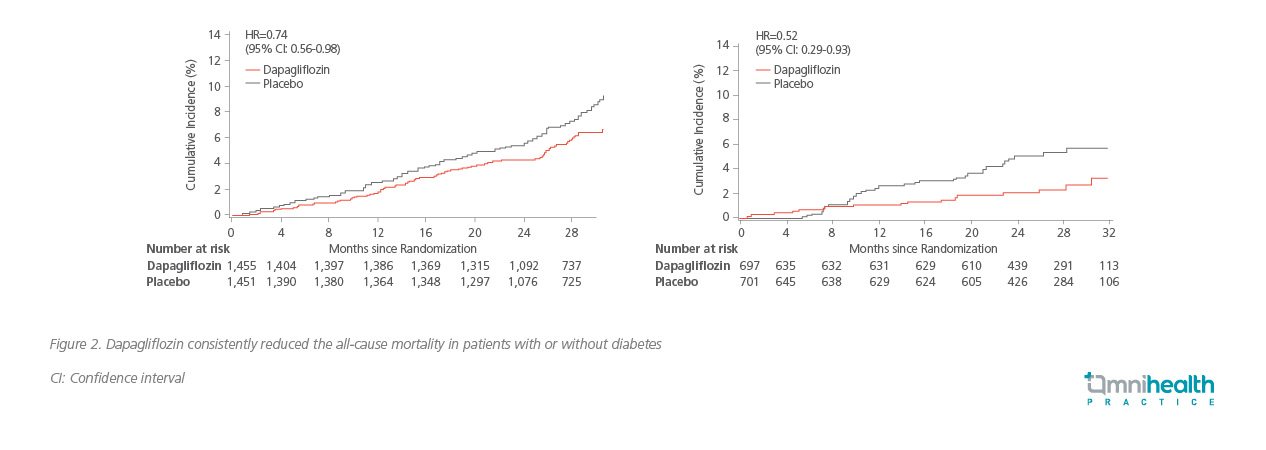
Managing cardiorenal complications in practice
Given the vast evidence supporting the cardiorenal benefit of SGLT2i, the Kidney Disease: Improving Global Outcomes (KDIGO) guideline now recommends SGLT2i and metformin as joint first-line treatment for patients with CKD, T2DM and an eGFR of ≥30mL/min/1.73m2.27 Importantly, SGLT2i initiation is particularly important for patients at high risk of CKD progression or HF as they derived the greatest absolute benefit with early treatment initiation.27
When prescribing SGLT2i, Prof. Pollock noted that SGLT2i are associated with a rare but increased risk diabetic ketoacidosis (DKA) and should be withheld on the day of day-stay surgery. During hospital recovery, blood glucose and ketone levels should be monitored and SGLT2i can be restarted after surgery when the patient can eat and drink normally. Given the diuretic nature of SGLT2i, diuretics should be spared when treating patients at risk of glycosuria to avoid dehydration. Otherwise, Prof. Pollock noted the significant cardiorenal benefits demonstrated by dapagliflozin in the DECLARE-TIMI 58, DAPA-CKD and DAPA-HF studies and urged all clinicians to consider SGLT2i early when treating patients at risk of cardiorenal outcomes.
Other than SGLT2i, finerenone, a selective mineralocorticoid receptor antagonist (MRA), also lowered the risk of CKD progression (HR=0.82; 95% CI: 0.73-0.93; p=0.001) and cardiovascular events (HR=0.86; 95% CI: 0.75-0.99; p=0.03) among patients with CKD and T2DM.28 Notably, finerenone was associated with a 31% greater reduction in UACR after 4 months of treatment (ratio of least squares=0.69; 95% CI: 0.66-0.71) that was maintained throughout the study.28 However, the combination of empagliflozin and MRA did not further improve cardiorenal outcomes, prompting the need for further investigation to confirm the benefit of this combination treatment.29
Conclusion
As the development of renal and cardiovascular dysfunctions are intricately linked, the treatment choice of CKD should account for both cardiovascular and renal outcomes. Where SGLT2i had demonstrated significant cardiorenal benefit in patients with CKD, dapagliflozin is the only drug supported by a totality of cardiorenal data in the DECLARE-TIMI 58, DAPA-CKD and DAPA-HF studies among patients with or without T2DM. To achieve evidence-based practice, Prof. Pollock recommends clinicians to adopt an SGLT2i that has documented kidney and cardiovascular benefits. “The adoption of optimal therapy is important when treating diabetic patients with CKD,” concluded Prof. Pollock.

