CONFERENCE UPDATE: EASD 2023
26-week treatment of dapagliflozin exhibits remarkable efficacy and tolerability among children and adolescents with T2D: Analysis of the phase 3 T2NOW study
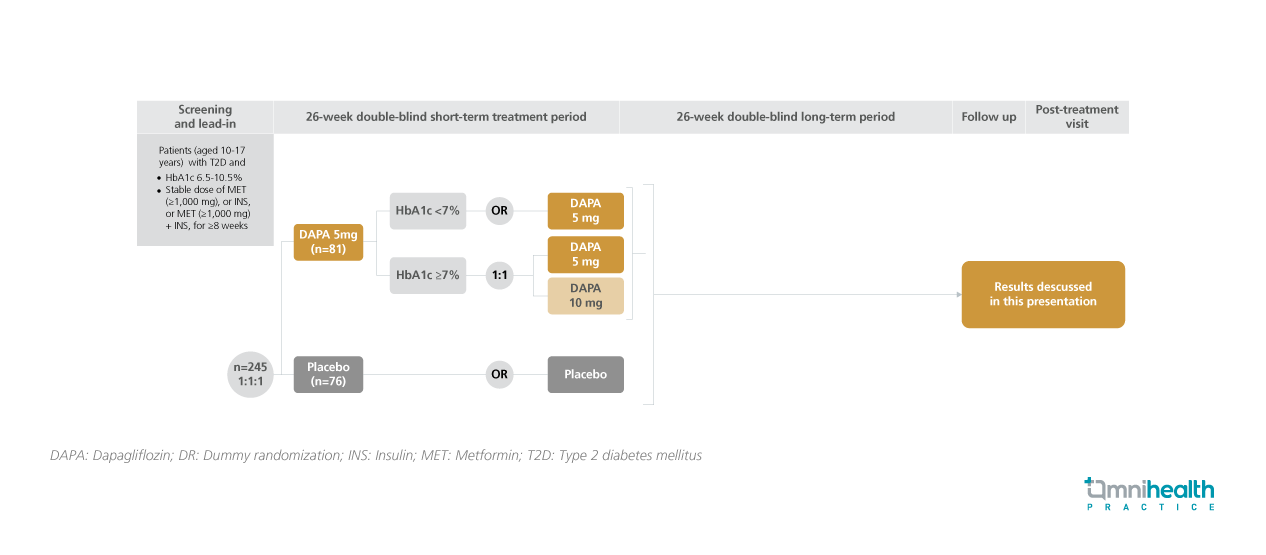
STUDY DESIGN
Despite an increasing incidence of type 2 diabetes mellitus (T2D) among children and adolescents, treatment options for this population remain limited.1 The T2NOW study was a 26-week, double-blinded, phase 3 study that investigated the efficacy and safety of dapagliflozin (DAPA) or saxagliptin versus placebo in children and adolescents with T2D.1 The clinical efficacy of DAPA among children and adolescents with T2D was reported in this analysis.1
In this study, T2D patients who were aged 10-17 years, had a hemoglobin A1c (HbA1c) level of 6.5-10.5% and received prior treatment of metformin and/or insulin for ≥8 weeks were randomized to receive a daily dose of DAPA 5mg (n=81) or placebo (n=76).1 The DAPA group was further assessed at Week 14 for their HbA1c levels.1 Patients with a HbA1c level <7% continued receiving dapagliflozin 5mg daily, whereas patients with a HbA1c level ≥7% were randomized to receive dapagliflozin 5mg or 10mg daily.1 After the primary endpoint assessment at week 26, the respective treatment of each group was continued until week 26.1
The primary endpoint of this analysis was the change in HbA1c levels from baseline at week 26.1 Secondary endpoints included safety, achievement rate of HbA1c <7% at week 26 and change in fasting plasma glucose (FPG) from baseline at week 26.1
| Primary endpoints: |
|
|
|
|
| Secondary endpoint: |
|
|
|
|
| Safety: |
|
|
|
“Dapagliflozin achieved the primary endpoint in this phase 3 study, confirming its efficacy and safety in children and adolescents with T2D receiving metformin, insulin, or both”
Dr. Naim Shehadeh
Institute of Diabetes, Endocrinology, and Metabolism,
Rambam Health Care Campus,
Haifa, Israel

