CONFERENCE UPDATE: EASD 2023
Semaglutide offers a sustained reduction in HbA1c and body weight in patients with T2D: Real-world data from the MHS database
STUDY DESIGN
Semaglutide has been shown to improve glycemic control, body weight, and cardiovascular outcomes among patients with type 2 diabetes (T2D) in randomized controlled trials and cohort studies.1 However, data on the long-term benefits of semaglutide in real-world settings has been lacking.1
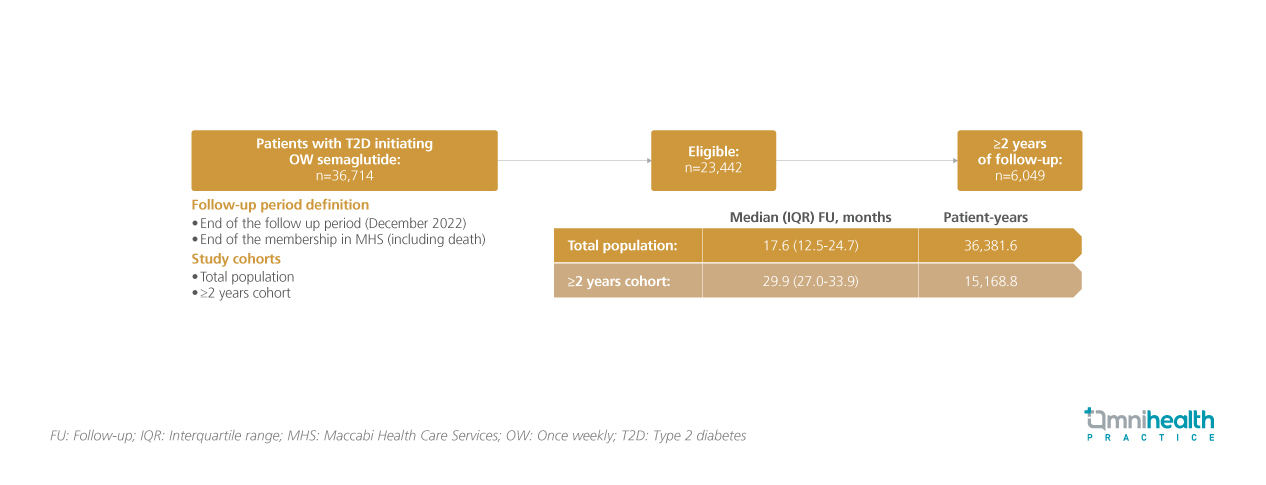
Therefore, a single-arm cohort study was conducted to assess the long-term effects of semaglutide treatment on HbA1c level, body weight, and other metabolic parameters in T2D patients treated for up to 3 years in the real world.1 This retrospective study included 23,442 T2D patients from the Maccabi Health Care Services (MHS) electronic database.1 Analysis was conducted in the total population (n=23,442) as well as the cohort of patients who had a follow-up of ≥2 years (n=6,049).1 The median follow-up was 17.6 months and 29.9 months in the total population cohort and the ≥2 years follow-up cohort, respectively.1
The primary endpoint of the study was the change in Hb1Ac from baseline to the end of the follow-up period. Key secondary endpoints included the change in body weight from baseline to the end of follow-up. The change in HbA1c and weight was also reported in subgroups stratified by baseline characteristics and in subgroups who were persistently on therapy. The exploratory outcome of change in HbA1c and weight after treatment discontinuation was also presented.
FINDINGS
| Primary endpoint: |
|
|
| Secondary endpoints: |
|
|
|
|
|
“The residual effect in both glycemic control and weight after semaglutide discontinuation merit additional investigation”
Dr. Cheli Melzer Cohen
Maccabi Institute for Research and Innovation,
Maccabi Healthcare Services,
Tel-Aviv, Israel

