CONFERENCE UPDATE
Semaglutide improves walking capacity and QoL in patients with symptomatic PAD and T2D: Results from the phase 3b STRIDE trial
STUDY DESIGN
Peripheral artery disease (PAD) is a common and severe complication of atherosclerotic vascular disease, particularly among diabetic patients.1 Despite the use of sodium-glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs) for type 2 diabetes (T2D), no treatments are specifically prioritized for their benefits in PAD, leaving patients with limited options and significant functional impairment.1
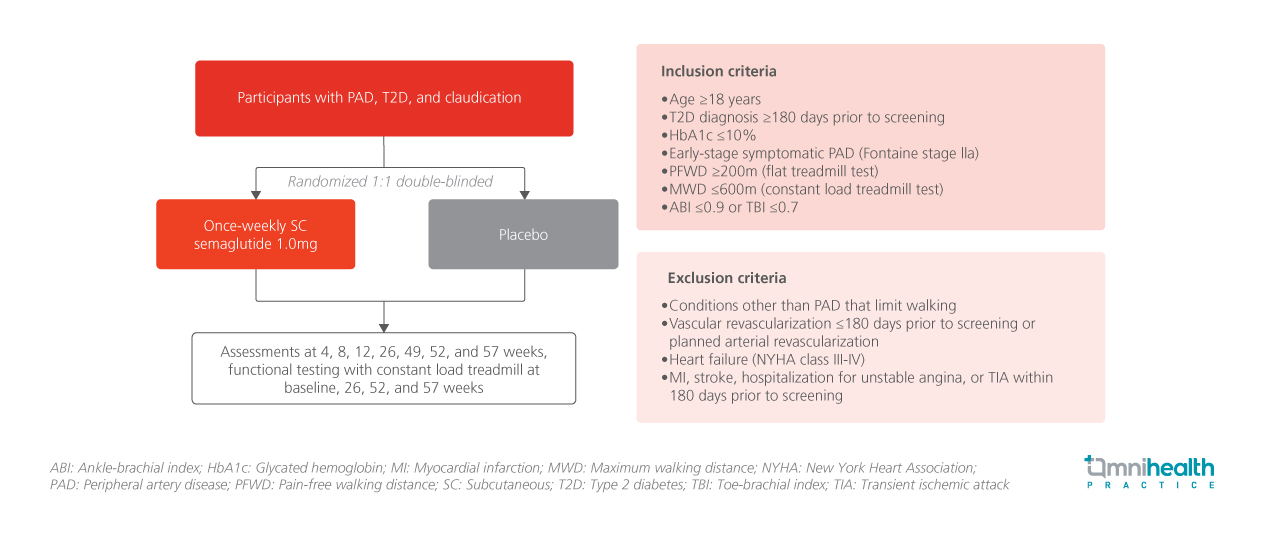
Semaglutide is a GLP-1RA approved for glycemic control in patients with T2D.1 In the phase 3b, double-blind, randomized, placebo-controlled STRIDE trial, the effect of once-weekly semaglutide on functional walking capacity, quality of life (QoL), and clinical outcomes in adults with T2D and PAD were evaluated.1 The trial enrolled 792 participants across 112 sites in 20 countries.1 Eligible patients had a diagnosis of T2D with HbA1c ≤10% and early-stage symptomatic PAD classified as Fontaine stage IIa, with a maximum walking distance (MWD) ≤600 meters on a constant load treadmill and pain-free walking distance (PFWD) ≥200 meters on the flat treadmill.1
Participants were randomized 1:1 to receive semaglutide 1.0mg (n=396) or placebo (n=396) subcutaneously once weekly for 52 weeks.1 Functional assessments were conducted at baseline, weeks 26, 52, and 57 using a constant-load treadmill with a fixed speed (3.2km/hr) and fixed inclination (12% grade).1 Baseline demographics and clinical characteristics were generally similar across the groups.1
The primary endpoint was the change from baseline (cfb) in MWD at week 52 (function).1 Confirmatory secondary endpoints included cfb in MWD at week 57 (function), cfb in PFWD at week 52 (function), and the change in VascuQoL-6 score from baseline to week 52 (QoL).1 Supportive secondary endpoints included cfb in the SF-36 physical function domain at week 52 (QoL), and the change in ankle-brachial index (ABI) from screening (week -2) to week 52 (hemodynamics).1
FINDINGS
| Primary endpoint: |
|
|
|
|
| Secondary endpoints: |
|
|
|
|
|
| Safety: |
|
|
|
|
"Semaglutide improves walking capacity, symptoms, and related QoL in patients with PAD and T2D"
Dr. Marc P. Bonaca
CPC Clinical Research,
University of Colorado,
Colorado, United States

