Insightful experience: SGLT2i to treat heart failure with reduced ejection fraction
Heart failure (HF) is global pandemic affecting at least 26 million people worldwide in 2017 and has been increasing in prevalence.1 In 2020, HF plus other heart diseases caused an age-standardized death rate of 34.0 per 100,000 standard population in Hong Kong.2 Previously, patients with HF with reduced ejection fraction (HFrEF) were treated with a foundational triple therapy including β-blockers, renin-angiotensin system inhibitors [including angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs)] or angiotensin receptor-neprilysin inhibitors (ARNIs), as well as mineralocorticoid receptor antagonists (MRAs).3 With reference to the recent data supporting the benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2is) in patients with chronic HFrEF regardless of the diabetes status, it is noted that comprehensive diseasemodifying quadruple therapy has now become the new standard of care for HFrEF patients.4,5 In a recent interview with Omnihealth Practice, Professor Siu, Chung-Wah David shared his insights on the application of SGLT2i in HFrEF treatment.
Recent updates on HF treatment guidelines
When HFrEF was first proposed to be a neurohumoral disorder in 1992, ACEIs were the only neurohumoral antagonists approved for treating chronic HF patients.6 Since then, the combination of ACEIs, β-blockers and MRAs has been regarded as the cornerstone of HFrEF treatment until further studies supporting the substitution of ACEIs with ARNIs.5 Recently, the clinical benefits of SGLT2i in treating HFrEF have been shown to be consistent regardless of the diabetes status, and supported SGLT2i as a new foundational therapy for HFrEF treatment.4,5 The latest 2021 American College of Cardiology (ACC) Expert Consensus, the 2021 European Society of Cardiology (ESC) guidelines, and the 2021 Canadian Cardiovascular Society/Canadian Heart Failure Society (CCS/CHFS) HF guidelines recommend the quadruple therapy of ACEIs or ARNIs, β-blockers, MRAs, and SGLT2i as the first-line treatment for all symptomatic HFrEF patients.7-9
Local experience of adopting quadruple therapy
In Hong Kong, when considering treatment for patients with stabilized, newly diagnosed HFrEF, the four drug classes in the quadruple treatment regimen are usually prescribed together instead of in sequence. Specifically, all four drug classes are usually initiated at a low dose and are gradually up-titrated altogether in the first 1 to 2 days, instead of titrating individual drug to a maximum dose before adding on another class of drug. Compared with other drug classes, it is noteworthy that SGLT2i is an exception as it uses only one fixed dose for HFrEF treatment and no up-titration is required.7
To physicians, it is sometimes difficult to require the patients, who have been discharged from the hospital, to follow strictly and increase the dose of a particular drug class of the quadruple therapy (except SGLT2i which requires no up-titration) to a maximum target dose. The recent consensus of the Heart Failure Association of the European Society of Cardiology suggested that all core therapies, except SGLT2i, can affect either blood pressure, heart rate or serum potassium level.10 If discharged to an outpatient setting, it may be difficult to monitor the patient’s dose adjustment closely and effectively, thus increasing the risk of side effects, such as hypotension, hyperkalemia, and low heart rate. In this regard, the equivalent starting and target dose of SGLT2i is highly convenient for initial HFrEF management. Also, having no significant effects on blood pressure and potassium level, patients taking SGLT2i are generally observed to have rapid improvements in symptoms, body weight and quality of life.
Upon using SGLT2i, an extra-low initial dose of other drug classes may be prescribed. Counseling advice on treatment should also be given for outpatient HFrEF management. Importantly, the overall principle of HFrEF treatment is to optimize quadruple guideline recommended therapies to the patients. As such, reducing the initial dose of ARNIs and β-blockers should be considered instead of taking no account of these drug classes.
SGLT2i’s consistent benefits to HFrEF patients with comorbid diabetes and CKD
Chronic kidney disease (CKD) is common in HFrEF, with a prevalence of 45% and is often associated with increased mortality and worsening of renal function in the HFrEF population.11 To address CKD, neurohormonal antagonists are usually prescribed and titrated based on the patient’s renal function and potassium level.11 While SGLT2i would be an effective and convenient treatment for HFrEF, dapagliflozin, in particular, has demonstrated the cardiovascular and renal protective benefits in CKD patients regardless of the diabetes status.12,13
In the phase 3, placebo-controlled, randomized DAPA-HF study involving 4,744 HFrEF patients who received a standard combination therapy, it was noted that the addition of dapagliflozin had significantly lowered the primary composite outcome of worsening HF (defined as an unplanned hospitalization or an urgent visit resulting in intravenous therapy for HF) or death from cardiovascular causes (16.3% in the dapagliflozin group vs. 21.2% in the placebo group; HR=0.74; 95% CI: 0.65-0.85; p<0.001) (figure 1).12 In particular, the DAPA-HF study had a similar number of patients with or without diabetes, and that dapagliflozin reduced the risk of death from cardiovascular causes (9.6% vs. 11.5%; HR=0.82; 95% CI: 0.69-0.98) and the risk of death from any causes (11.6% vs. 13.9%; HR=0.83; 95% CI: 0.71-0.97) when compared with placebo among both populations.12 In terms of safety, the frequency of adverse events (AEs) related to volume depletion, renal AEs, and major hypoglycemia, was not statistically different between the dapagliflozin group and the placebo group.12
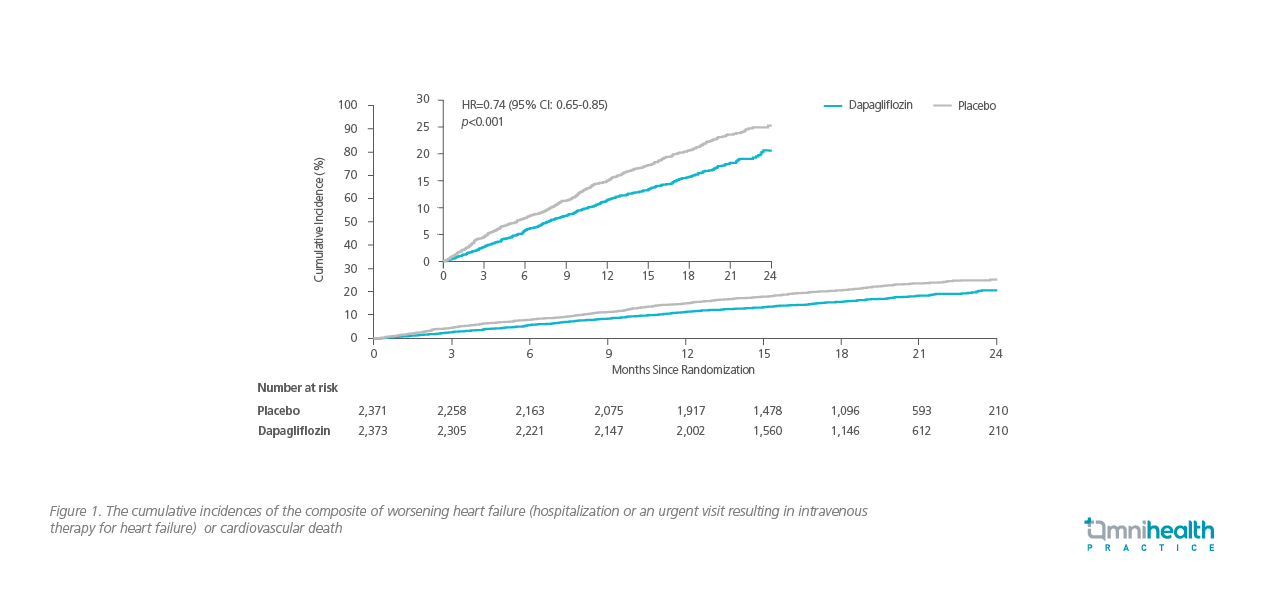
In addition to the cardiovascular benefits, renal protection is equally critical for HF treatment as approximately half of the HFrEF patients also suffer from CKD.11 In the randomized, doubleblind, placebo-controlled DAPA-CKD study, 4,304 participants with an estimated glomerular filtration rate (eGFR) of 25 to 75mL/min/1.73m2 of body-surface area and a urinary albuminto- creatinine ratio (UACR) of 200 to 5,000 were randomized to receive either dapagliflozin or placebo.13 Compared with placebo, dapagliflozin significantly reduced the risk of the composite of sustained decline in eGFR of at least 50%, end-stage kidney disease or death from renal or cardiovascular causes (9.2% in the dapagliflozin group vs. 14.5% in the placebo group; HR=0.61; 95% CI: 0.51-0.72; p<0.001) (figure 2), as well as death from any causes (HR=0.69; 95% CI: 0.53-0.88; p=0.004).13
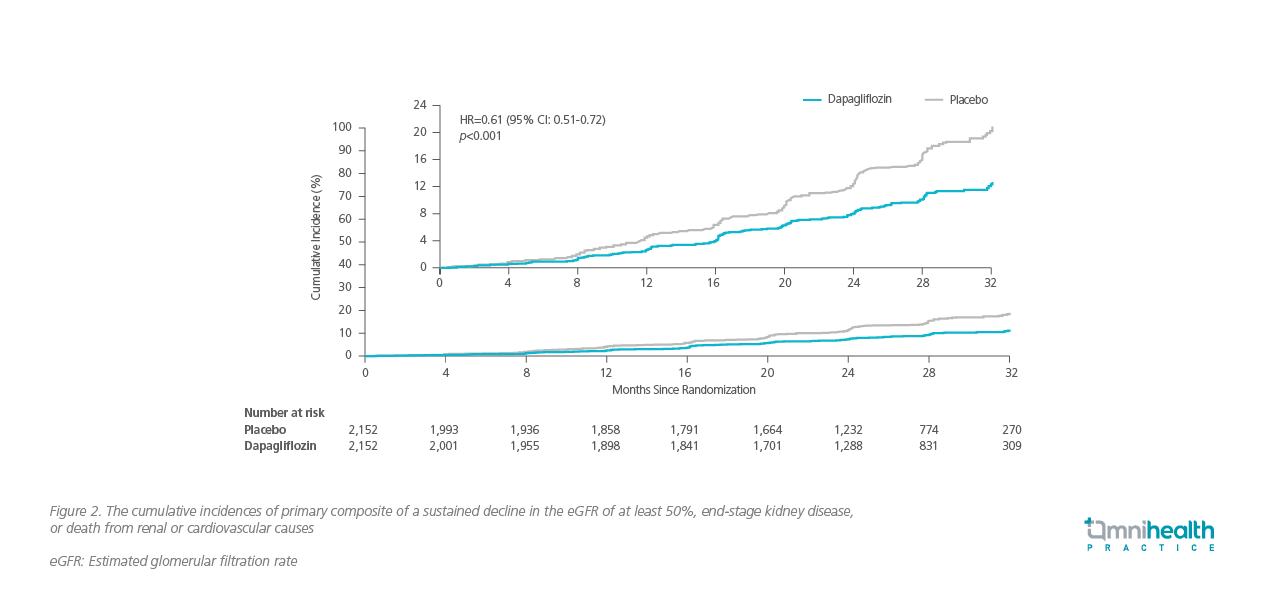
Of note, the DAPA-CKD trial confirmed that the kidney protective benefits of dapagliflozin could be extended to the broader population of CKD patients with or without type 2 diabetes, for whom ACEIs were the only pharmacologic treatments that were shown to prevent kidney failure.13 Importantly, dapagliflozin slowed down the decline of eGFR from baseline to 30 months of treatment, with a slope of -2.86 vs. -3.79mL/min/1.73m2 per year, resulting in inter-group difference of 0.93mL/min/1.73m2 per year in the long-term (95% CI: 0.61-1.25) (figure 3).13 Similar to the DAPA-HF trial, the benefits of dapagliflozin were comparable in patients with or without type 2 diabetes, with no new safety signals were identified.13

Applying SGLT2i to HFrEF patients with CKD
As β-blockers do not provide obvious renal benefits and SGLT2i demonstrated a delay in CKD progression, it is reasonable to initiate dapagliflozin in patients with renal dysfunction or CKD in the early course of HFrEF. However, due to its diuretic nature, SGLT2i should be used in caution in the elderly population who is often at a high risk of genital infections. Caregivers should also be aware of the importance of maintaining adequate hydration, especially for those elderly patients. Besides, it is important to increase patients’ tendency of adopting SGLT2i to maintain treatment compliance to achieve adequate control of HFrEF.
There have been concerns regarding the association of euglycemic diabetic ketoacidosis (eDKA) with SGLT2i.14 However, SGLT2irelated eDKA cases are rare with a reported incidence of 0.16 to 0.76 events per 1,000 patient-years in patients with type 2 diabetes.15 In DAPA-HF, the reported rate of diabetic ketoacidosis was very low (3 cases out of 2,368 patients in the dapagliflozin group vs. 0 in the placebo group), and all cases occurred only in patients with diabetes at baseline.12 Although the survival benefits of SGLT2i on HFrEF patients outweigh their minor risk of eDKA, physicians should still be alert of the symptoms of eDKA among patients on SGLT2i treatment.14 Typical signs and symptoms of eDKA include nausea, vomiting, abdominal pain, dyspnea, lethargy, and dehydration.14
A message to physicians
Evolving from ACEI to SGLT2i, advances in HF treatment over the past 30 years have reduced the mortality risk of HFrEF patients. Yet, most of these treatment options still require dose adjustment throughout the course of disease. As an essential part of the quadruple therapy recommended by the international guidelines, dapagliflozin has demonstrated the cardiovascular and renal protective benefits in both HFrEF and CKD patients regardless of the diabetes status. Without the need of dose adjustment, dapagliflozin should be considered early in the HFrEF treatment for better disease control. Notwithstanding this, accumulated clinical experience is required in order to translate the benefits of medications from clinical trials to practical use.
Conclusion
HFrEF is a debilitating chronic illness contributing to substantial cardiomyocyte loss and systolic dysfunction. With the promising efficacy and safety of dapagliflozin demonstrated in HFrEF and CKD patients regardless of the diabetic status, the recent updated guidelines recommend adding SGLT2is to ARNIs, MRAs and β-blockers as a new quadruple standard of care for HFrEF management. Despite concerns of SGLT2i-induced eDKA in patients with HFrEF, which is rare indeed, it should not overrule the benefits of SGLT2i and delay the decision of adding SGLT2i to the guideline-directed medical therapy.

