CONFERENCE UPDATE: EASD 2023
Empagliflozin as an efficacious novel oral option for youth-onset type 2 diabetes patients without insulin therapy: Post-hoc analysis of the DINAMO trial
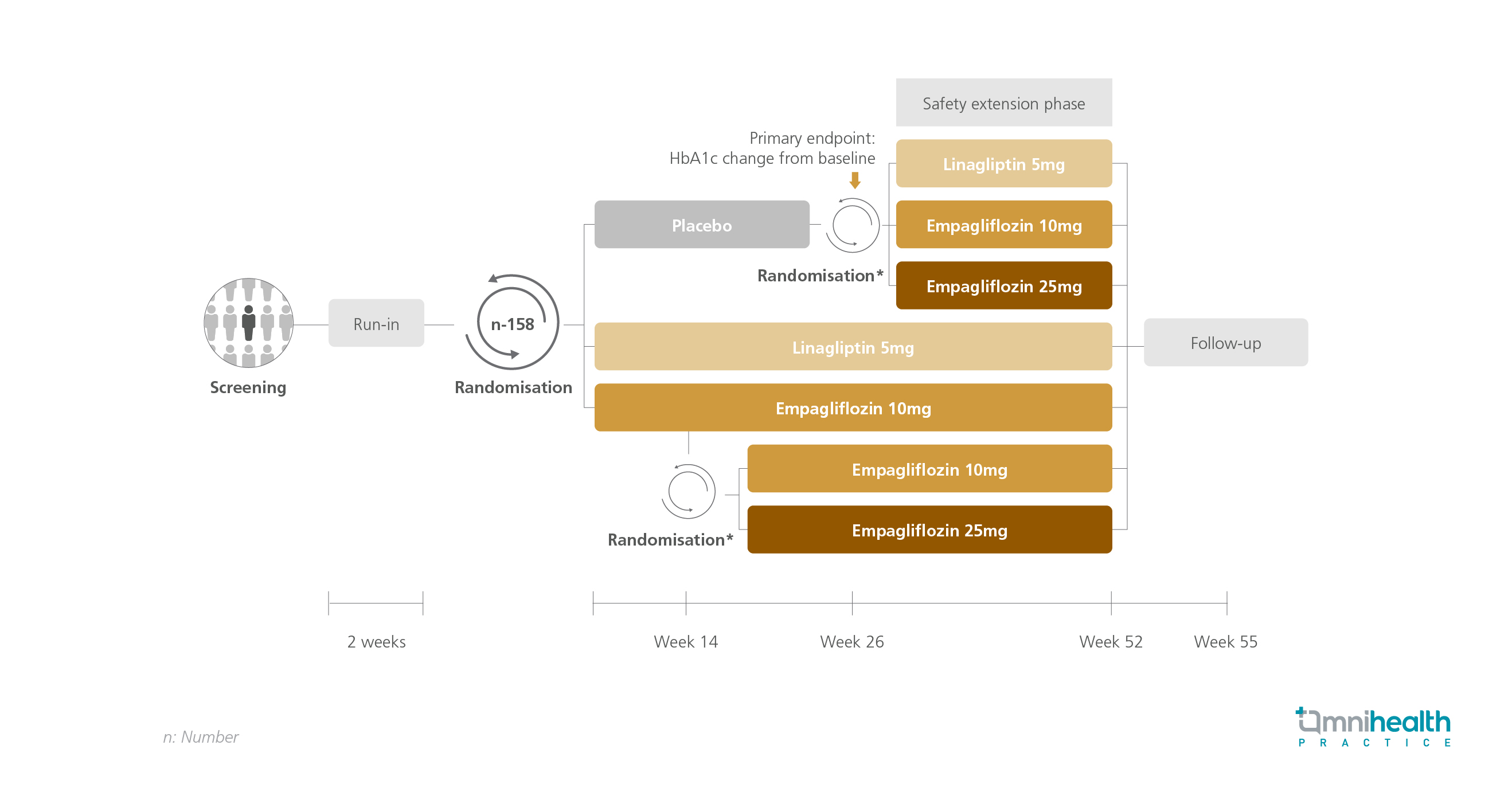
STUDY DESIGN
Compared to their adult counterparts, children and adolescents with type 2 diabetes mellitus (T2DM) experience an accelerated deterioration in beta-cell function and development of insulin resistance, thereby elevating their risk of early-onset complications. Currently, treatment options for young-onset T2DM are limited to injections and metformin, the only globally approved oral agent.1 To assess the efficacy and safety of empagliflozin and linagliptin in young-onset T2DM, the DINAMO trial, a randomized, double-blind, placebo-controlled study, was conducted.1 A subsequent post-hoc analysis was commenced to evaluate the effects of background insulin therapy on the treatment outcomes of empagliflozin and linagliptin.
The DINAMO trial enrolled a total of 158 children and adolescents with T2DM, upon which 43.3% of patients were prescribed insulin therapy. The study population (n=158) was randomized to placebo, linagliptin 5mg, or empagliflozin 10mg.1 At week 12, patients who failed to reach a hemoglobin A1c (HbA1c) level of <7% in the empagliflozin 10mg arm were further randomized into either continuing their treatment or receiving empagliflozin 25mg instead.1 After the primary endpoint analysis at week 26, patients from the placebo arm were also randomized into receiving linagliptin 5mg, empagliflozin 10mg, or empagliflozin 25mg.1
The primary endpoints of this post-hoc analysis were the adjusted mean changes in HbA1c and fasting plasma glucose (FPG) levels from baseline to week 26 and week 52.1
| Primary endpoints: |
|
|
|
|
|
|
“These results support the use of empagliflozin as a new oral treatment option in young people with T2DM, delaying the need for insulin therapy”
Dr. Igor Tartakovsky
Boehringer Ingelheim International GmbH,
Ingelheim, Germany

