CONFERENCE UPDATE: ACC 2024
Weight and HF outcomes in obese HFpEF and T2DM patients with semaglutide in the STEP-HFpEF DM trial
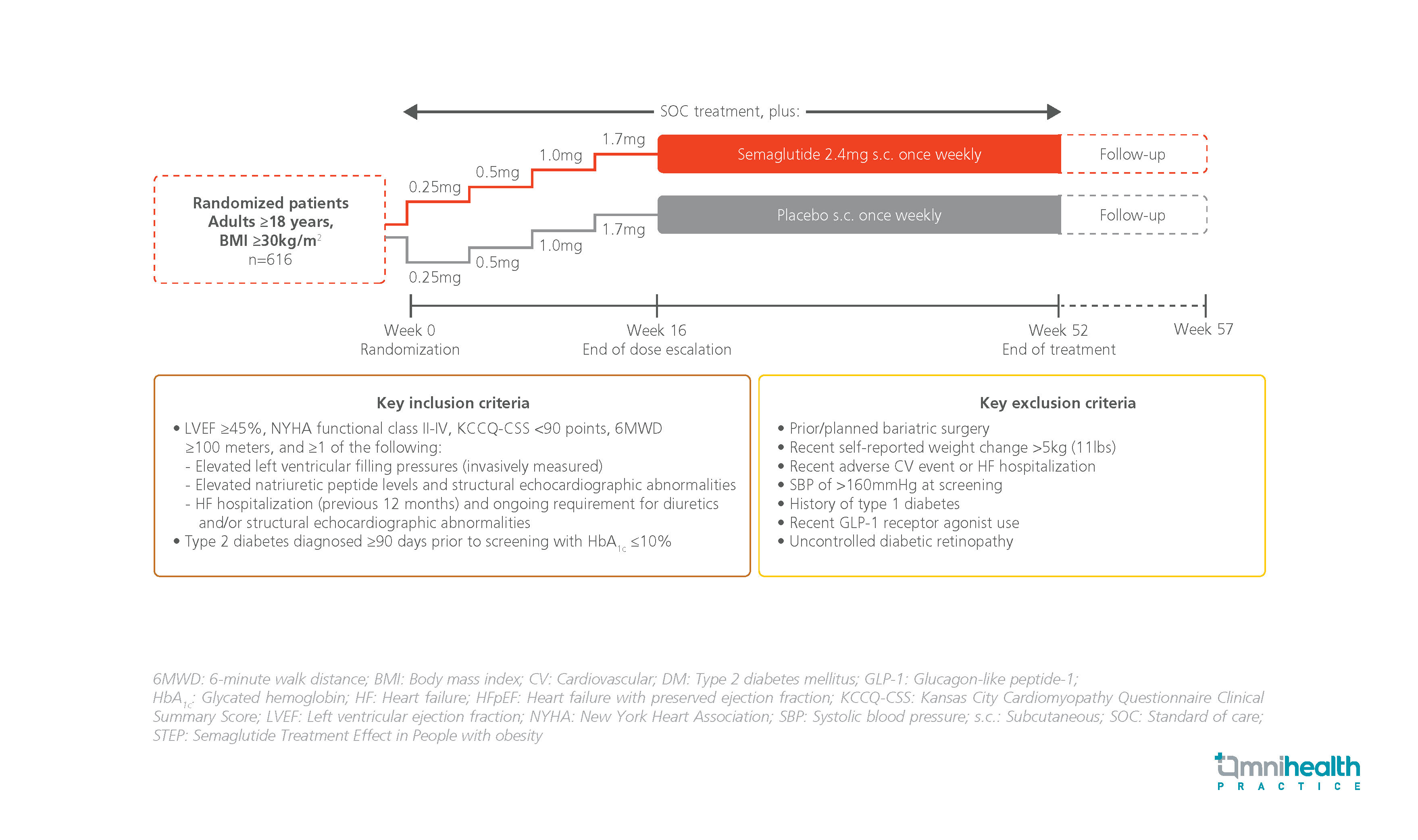
STUDY DESIGN
Excessive adiposity is associated with the development and progression of both heart failure with preserved ejection fraction (HFpEF) and type 2 diabetes mellitus (T2DM).1 Comorbidity of T2DM in patients with HFpEF is common, resulting in a greater symptom burden and worse functional status.1 Semaglutide is a once-weekly glucagon-like peptide-1 receptor agonist (GLP-1RA) shown to significantly improve heart failure (HF)-related symptoms, physical limitations and body weight in patients with obesity-related HFpEF without T2DM.1
The STEP-HFpEF DM trial is the first randomized, double-blind, placebo-controlled phase 3 trial to investigate the efficacy of once-weekly semaglutide in patients with obesity-related HFpEF and T2DM.1 The study included adult patients with body mass index (BMI) ≥30kg/m2.1 They had T2DM and class II-IV HF by the New York Heart Association (NYHA) functional classification, a left ventricular ejection fraction (LVEF) ≥45%, <90 points on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), and a 6-minute walking distance test (6MWD) result of ≥100 meters.1 Patients were randomized 1:1 to receive the standard of care plus either subcutaneous semaglutide 2.4mg (n=310) or a matching placebo (n=310) once weekly for 52 weeks.1
The primary endpoints were the change in KCCQ-CSS and the percentage change in body weight from baseline to week 52.1 Secondary endpoints included the change in 6MWD and change in C-reactive protein (CRP) levels from baseline to week 52.1 Hierarchical composite endpoint which comprised time to all-cause mortality, number and time to HF events requiring hospitalization, and differences in KCCQ-CSS change and 6MWD from baseline to week 52 was also assessed.1
|
Primary endpoints: |
|
|
Secondary endpoints: |
|
|
Safety: |
|
“In patients with obesity-related HFpEF and T2DM, semaglutide 2.4mg produced larger reductions in HF-related symptoms and physical limitations, greater weight loss, and greater improvements in exercise function than placebo”
Dr. Mikhail N. Kosiborod
Department of Cardiovascular Disease,
University of Missouri-Kansas City School of Medicine,
Kansas City, United States

