Case Review
Local case series: Optimizing long-term management of RRMM with all-oral ixazomib regimen
Multiple myeloma (MM) remains an incurable disease, and nearly all patients will have a relapse at some point in their life.1 The recent advance in MM management has brought about significant improvements in the survival of MM patients.1 Thanks to the prolonged survivals, clinicians increasingly consider drug tolerability, patient adherence and quality of life (QoL) important in MM management.2 In 2018, the Department of Health approved ixazomib in combination with lenalidomide and dexamethasone (IRd), for the treatment of patients with MM who failed one prior therapy [i.e., relapsed/refractory MM (RRMM)].3 The ixazomib-based therapy was the first-and-only, all-oral RRMM regimen which helps delay disease relapse among MM patients while improving treatment adherence and maintaining their QoL.1,4 In the interviews with Omnihealth Practice, Dr. Liu, Sung-Yu Herman, Dr. Au, Wing-Yan Thomas and Dr. Wong, Siu-Ming Raymond adopted the all-oral regimen in their practice and shared 3 clinical cases in which the use of IRd in 3 RRMM patients of different patient characteristics was well tolerated and achieved good clinical responses.
Background
MM is a hematologic disorder which is associated with a proliferation of malignant, monoclonal plasma cells in the bone marrow and/or extramedullary sites.5 In China, a recent study showed the prevalence and incidence of MM were 5.68 and 1.15 cases per 100,000 people, respectively, with the disease being more common among females (p<0.05).6 The disease is mostly seen among people aged between 55-74 years, with the median age of MM patients being 70 years at diagnosis.6,7 Due to the aging population, the prevalence of MM is expected to rise significantly.8
The typical manifestations of MM include end-organ damage including hypercalcemia, renal impairment, anemia and lytic bone lesions, collectively known as CRAB symptoms.5 Apart from the potential end-organ damage, MM also significantly affects patient health-related QoL (HRQoL), including reduced physical functioning, fatigue, and pain.4
The treatment goals of MM include relieving disease symptoms, preventing new organ damage and delaying another disease relapse.5 Intensive induction therapy followed by autologous stem cell transplant (ASCT) is the current standard of care for MM patients.8 However, randomized trials found that <20% of patients aged >65 years received ASCT despite the demonstrated survival benefits.7 Bortezomib-based triplet regimens have demonstrated efficacy among these transplant-ineligible MM patients as the first-line therapy.9 Nevertheless, nearly all patients with MM will experience disease relapse at some point of their diseases despite the initial therapy and require further treatment.1
The continuous treatment paradigm of MM
Over the past 15 years, the introduction of proteasome inhibitors (PI) and immunomodulatory drugs has markedly improved the survival of MM patients, with the median overall survival (OS) increasing from 4.6 years among patients diagnosed with MM between 2001 and 2005 to 6.1 years between 2006 and 2010.9 Longer survival has meant that more and more patients are candidates for subsequent lines of therapies.10 In recent years, continuous therapy has emerged as a new standard treatment paradigm in MM management, having demonstrated superior outcomes vs. fixed-duration therapy.9 More specifically, a prolonged PI-based regimen is desirable since many studies have shown that continuous PI-based treatment is associated with improved outcomes vs. shorter PI-based therapy.9
While long-term, continuous treatment is increasingly utilized, the fact that MM patients are generally older and are more prone to treatment-related toxicities could limit its use.9 For example, continuous treatment with bortezomib- or carfilzomib-based regimens could be difficult to achieve due to peripheral neuropathy (PN), cardiovascular (CV) and renal toxicities.9 Therefore, clinicians need to be cautious in deciding the treatment in each line since early discontinuation of therapy due to toxicity will limit the options in the later lines of treatment.9 A well-tolerated PI-based regimen with few late-onset or cumulative toxicities that can be administered over an extended period of time is very much needed.9
Ixazomib: The first all-oral PI-based regimen
Ixazomib is the first oral PI which has been approved, in combination with Rd, for the treatment of MM patients who have received at least one prior therapy.9 The approval was based on the positive results of the TOURMALINE-MM1 study (n=722), the first phase 3 study with a placebo-controlled, double-blinded study design to evaluate the safety and efficacy of PI-based triplet regimen in RRMM.9 With a median follow-up of 14.7 months, IRd significantly reduced the risk of disease progression by 26% (HR=0.74; 95% CI: 0.59-0.94; p=0.01) and prolonged progression-free survival (PFS) by 5.9 months (20.6 months vs. 14.7 months) when compared with the placebo, Rd alone (figure 1).11 The overall response rate (ORR) of IRd was also significantly higher than that of Rd (78.3% vs. 71.5%; p=0.04).11 Treatment response was rapid, durable and deepened over time.12 An increasing number of participants was found to achieve a very good partial response (PR) or a complete response (CR) when they stayed longer in IRd treatment.11 Notably, about 48% of patients were able to receive ≥18 cycles of IRd, with some patients receiving up to 34 cycles.9,11 The results demonstrated the feasibility of long-term administration of IRd among RRMM patients and the important role of IRd in the new continuous treatment paradigm of MM.
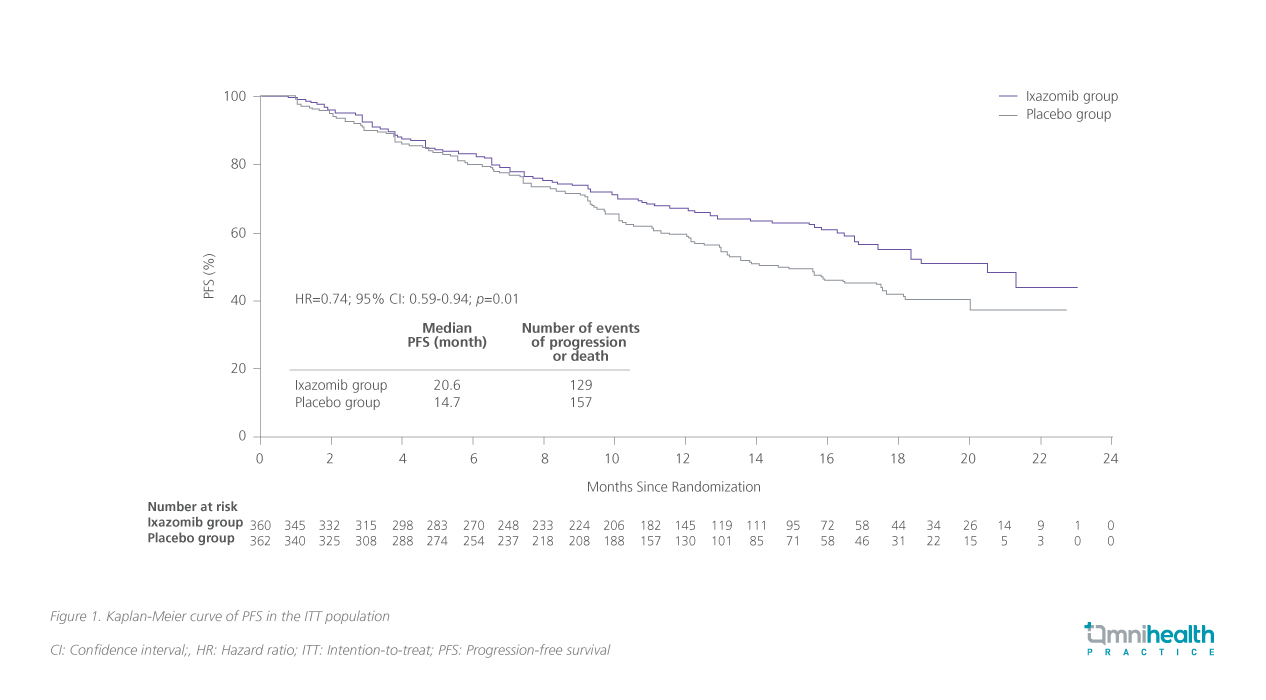
In addition, TOURMALINE-MM1 also showed that the efficacy of IRd was not affected by the advanced age and frailty of older RRMM patients, as evidenced by the similar PFSs achieved by different age subgroups (20.6 months for those aged ≤65 years; 17.5 months for those aged >65-75 years; 18.5 months for those aged >75 years).11 This is probably due to IRd’s convenient oral administration, the unnecessity to adjust the dose among patients with mild-to-moderate renal impairment, as well as its favorable and easily manageable safety profile.9
Recognizing its benefits, IRd was widely adopted by hematologists in the local practice to help MM patients to stay in treatment and improve their QoL. The below 3 local clinical cases, shared by Dr. Liu, Dr. Au and Dr. Wong, demonstrated the usefulness and clinical benefits of ixazomib-based therapy among local RRMM patients with different baseline characteristics. These cases are believed to be able to provide insights and practical tips for other hematologists to better manage their patients and optimize the treatment outcomes with IRd.
Case 1 from Dr. Liu, Sung-Yu Herman
A 78-year-old female complained of lower back pain and was subsequently diagnosed with Revised-International Staging System (RISS) stage 2 MM with t(4;14) translocation in 2015. She had a past medical history of hypertension, atrial fibrillation (AF) and a collapsed L1 vertebra. Despite the comorbidities and symptoms, she had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 1. She was found to have mild renal impairment with creatinine clearance of 80mL/min and mild anemia with a serum hemoglobin level of 8.5g/dL. She did not have extramedullary disease, and her serum calcium level was normal. Due to her advanced age, she was not eligible for bone marrow transplant.
The patient was treated with upfront bortezomib, thalidomide and dexamethasone (VTd) regimen for 4 cycles, achieving good responses. Her serum paraprotein level became undetectable, and her anemia also resolved. The patient remained in remission for 2.5 years with lenalidomide as maintenance therapy.
In 2019, regular biochemistry identified a steady increase in serum paraprotein from an undetectable level to 10g/L in 3 months. The patient was asymptomatic, and further examinations found no hypercalcemia or other end-organ damage.
The all-oral IRd regimen was utilized to manage her biochemical relapse. In just 3 months, her serum paraprotein dropped from 11g/L to an undetectable level. She has remained in remission since then. During the treatment course of IRd, the patient experienced mild rash in the first cycle, which was effectively managed with topical hydrocortisone 1% cream. The rash subsided 3 days later and did not recur. The patient did not experience diarrhea or PN or hematological toxicities such as neutropenia throughout the treatment.
Discussion
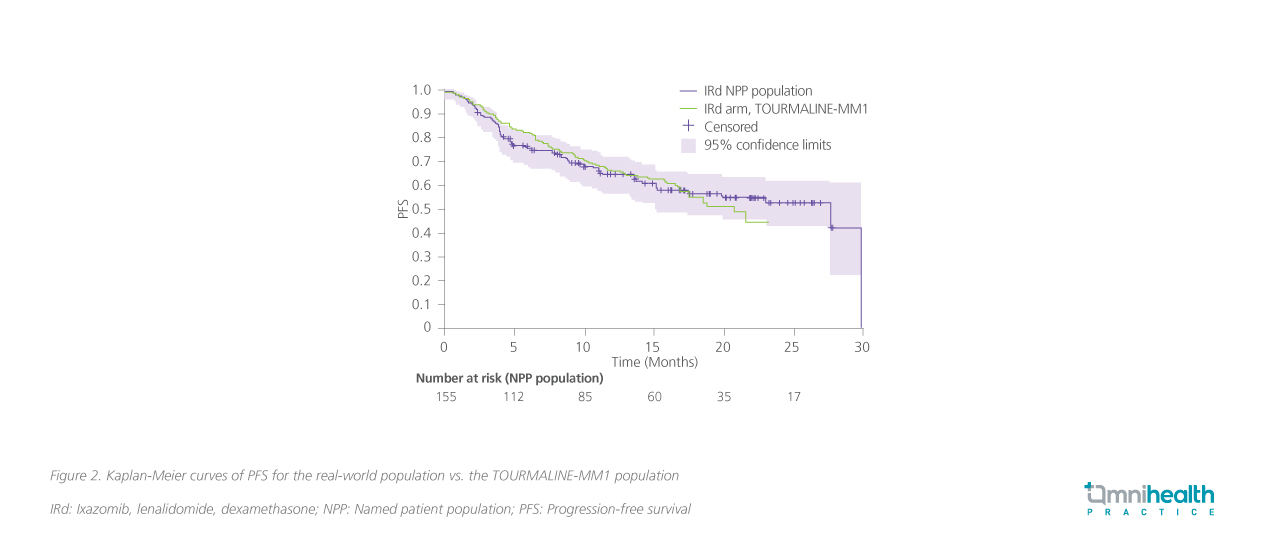
The TOURMALINE-MM1 study has demonstrated the PFS benefits and limited additional toxicity with the addition of ixazomib to Rd among patients with RRMM.11 To evaluate the safety and the therapeutic effect of IRd in the non-trial setting, a number of real-world studies across the world were conducted, including a multi-center, retrospective, observational study with patients from the United Kingdom (UK), Greece and Czech Republic.12-14
In this study, a total of 155 adult patients with RRMM who had received 1-3 prior lines of therapy (LoT) and at least one cycle of IRd were included, in accordance with the eligibility criteria for the TOURMALINE-MM1 study.12 The median age was 68.1 years, and about one-sixth (17%) of patients had an ECOG PS of ≥2.12 Majority of patients (91%) had been exposed to bortezomib, and about half had received thalidomide (47%).12 The median follow-up for this analysis on PFS was 18.8 months.12
Results showed that among patients who had received IRd as second-line, the median PFS was 27.6 months (95% CI: 15.1-29.8) (figure 2).12 Those receiving IRd as ≥3 lines had a median PFS of 19.9 months (95% CI: 13.7-not estimable).12 The ORR was 73.8%, with >50% of patients achieving PR or very good PR (VGPR) (58.1%).12 To conclude, the findings of this real-world study were consistent with the results of the TOURMALINE-MM1 trial.
Dr. Liu looked into a few factors while deciding on the treatment for this patient, especially the cardiotoxicity associated with the use of PI. He suggested avoiding treatment options with irreversible proteasome inhibition properties and a higher reported incidence of CV diseases.15 On the other hand, oral ixazomib was reported to have a low incidence of CV events (1.3%) and therefore was preferred in this patient.15 Dr. Liu reminded that MM patients are generally older, and more than 50% of them are aged >65 years.15 Older patients are more prone to cardiotoxicity associated with PI since they have markedly reduced ubiquitin-proteasome pathway (UPP) activity and consequently increased proteasome instability.15 In addition, most elderly patients have CV risk factors, and patients with MM often have prior exposure to cardiotoxic therapies such as anthracyclines or chest radiotherapy, further increasing the likelihood of PI-associated cardiotoxicity.15 Thus, PI with a lower risk of CV events is a more favorable treatment option for older patients and those with baseline CV diseases.
Another factor was patient’s preference. In recent years, the inclusion of patient preferences in decision-making has been widely promoted around the world to improve overall treatment outcomes.16 According to a study from the United States (US), patients are generally averse to severe nerve damage, which could seriously limit normal daily activities and compromise patients’ QoL.16 Among the PIs, bortezomib-based therapy was associated with a higher incidence of PN.17 Ixazomib (i.e., IRd), in contrast, showed a notably reduced incidence of PN, with ≥3 PN occurring in only 2% of patients.17 In addition, studies also reflected patients’ preference for oral administration over injection.16 A survey found that patients treated with oral therapy reported greater treatment effectiveness, satisfaction and convenience vs. injection.16 “I think patients are satisfied with the all-oral regimen since they can have less frequent hospital visits and experience fewer adverse events”, Dr. Liu said.
Take-home message
Overall, Dr. Liu emphasized that RRMM therapy should be decided in a shared decision-making framework, catering as much as possible to the needs of the patients. Patients’ treatment preferences must be taken into account since they could affect their treatment compliance and long-term outcomes. Baseline patients' characteristics, especially CV diseases, ought to be taken into account. “The all-oral regimen is very convenient to patients, and the response is rapid and durable. I would prefer this over conventional intravenous (IV) regimen in suitable patients,” Dr. Liu recommended.
"I think patients are satisfied with the all-oral regimen since they can have less frequent hospital visits and experience fewer adverse events."
Dr. Liu, Sung-Yu Herman
Specialist in Hematology & Hematological Oncology
Case 2 – from Dr. Au, Wing-Yan Thomas
A 79-year-old male was diagnosed with MM in 2017. He had CV comorbidities and had previously received percutaneous coronary intervention (PCI). He also had Parkinson’s disease. Initial diagnostic workup found 50% plasma cell in the bone marrow and a high immunoglobin A (IgA) concentration of 4,800mg/dL. He was also found to have extramedullary disease and carry a high cytogenetic risk.
The patient was managed upfront with IV bortezomib for 2 months, achieving no response. Then, carfilzomib was administered for 4 cycles, decreasing the myeloma cell from 55% to 36% and IgA concentration from 4,800mg/dL to 1,500mg/dL. Since the patient could not tolerate continued IV treatments, he was switched to the all-oral ixazomib-based regimen in February 2018. The patient had no progression since then and had been on ixazomib treatment for more than 4 years. Throughout the treatment, the patient did not experience any adverse events (AEs) associated with ixazomib.
Discussion
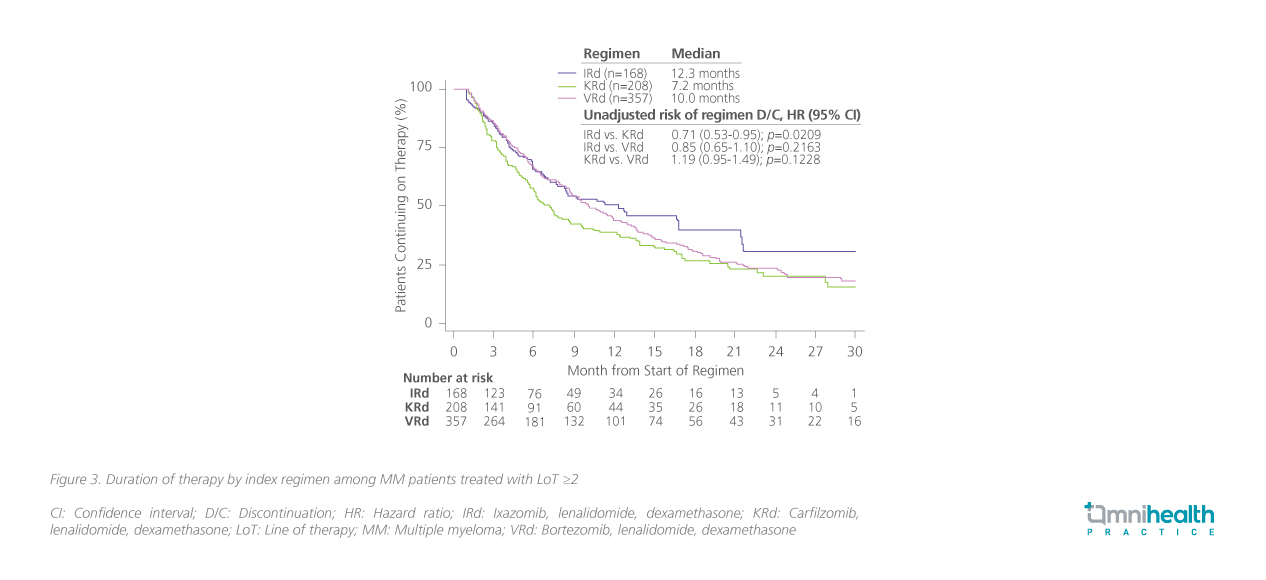
In the RRMM setting, there have been several PI-based triplet combinations that have demonstrated superior efficacy over the Rd doublet regimen in the clinical trials, offering more viable options to patients for prolonging their survival.14 These regimens include carfilzomib with lenalidomide and dexamethasone (KRd), IRd and VRd. Due to the lack of head-to-head trials and differences in the trial populations, it remains a challenge to compare the treatment effects of these combinations, especially among real-world RRMM patients who generally have a higher comorbidity burden and are mostly not eligible for clinical trials.14
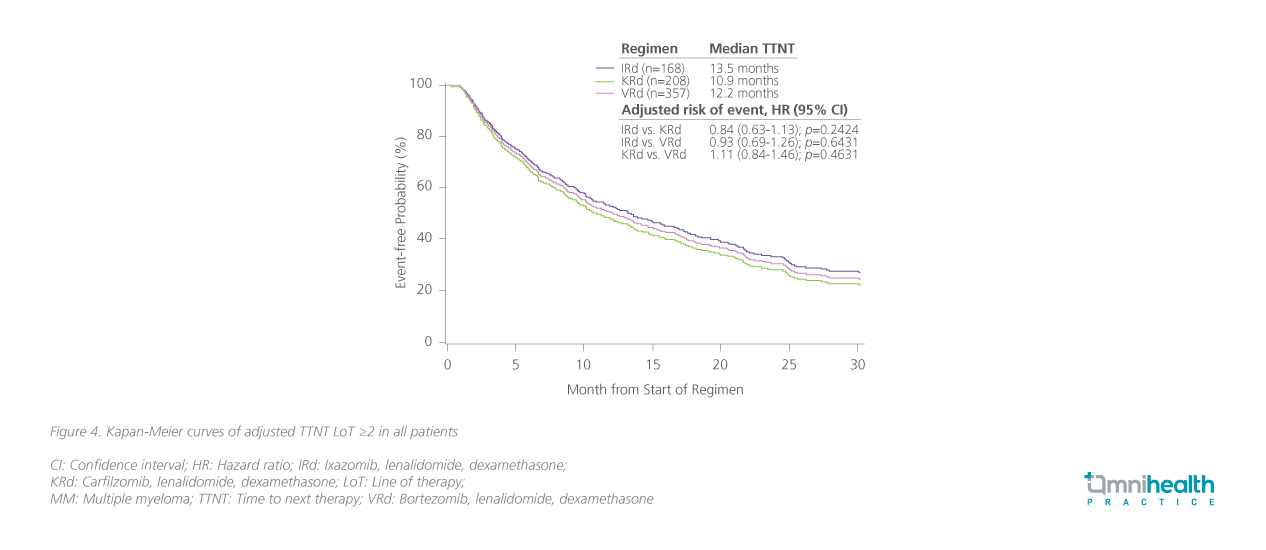
A US retrospective cohort study was therefore conducted, including 64% of patients treated with IRd (n=168; median age=71 years), KRd (n=208; median age=65 years) or VRd (357; median age=71 years) in ≥2 LoT.14 Patients treated with IRd were more likely to have high-risk cytogenetics (OR=1.96; 95% CI: 1.14-3.38; p=0.0152) and prior immune-mediated inflammatory disease (IMID) exposure (OR=4.34; 95% CI: 2.52-7.49; p<0.0001).14 Results showed that the median duration therapy was longest with IRd (12.3 months), followed by VRd of 10 months, and KRd of 7.2 months.14 The risk of treatment discontinuation was significantly lowered with IRd vs. KRd (HR=0.71; 95% CI: 0.53-0.95; p=0.0209) (figure 3).14 On the other hand, patients treated with IRd had an adjusted time to next therapy (TTNT) of 13.5 months, comparable to 12.2 months with VRd and 10.9 months with KRd (figure 4).14 Without adjustment for covariates, patients treated with IRd had a lower risk of initiation of next LoT or death vs. KRd (HR=0.76; 95% CI: 0.58-1.00; p=0.047).14
Take-home messages
When deciding regimens for patients with disease relapse, Dr. Au stressed the importance of considering the previous LoT, treatment responses and prior AEs.2 Age, frailty status and patients’ expectations should also be taken into account.2 Since treatment-related AEs are a frequent cause of premature discontinuation, which will negatively affect long-term outcomes. Since this patient was very old and relatively frail, a well-tolerated and convenient regimen was desired. In TOURMALINE-MM1, IRd was shown to be well tolerated, with most AEs being primarily grade 1 or 2 diarrhea, neutropenia and anemia. Also supported by real-world evidence, the use of IRd was believed to be long-lasting and effective in delaying the next LoT. “Tolerability is a particularly important consideration in the management of RRMM. Patients need to stay in each line of treatment as long as possible to achieve optimal long-term outcomes,” Dr. Au concluded.
"Tolerability is a particularly important consideration in the management of RRMM. Patients need to stay in each line of treatment as long as possible to achieve optimal long-term outcomes."
Dr. Au, Wing-Yan Thomas
Specialist in Hematology & Hematological Oncology
Case 3 – from Dr. Wong, Siu-Ming Raymond
A 78-year-old female with myasthenia gravis (MG) sought medical attention due to mild anemia with hemoglobin level of 11g/L in 2013. However, the initial diagnostic workup revealed a reversed albumin globulin ratio and a high serum paraprotein level of 28g/L. Bone marrow aspiration found 70% of plasma cells in the bone marrow, and fluorescence in situ hybridization (FISH) showed 1q21 amplification. Her renal function and calcium level were normal. There were no bone lesions on the skeletal survey. The patient was confirmed with a diagnosis of MM without end-organ damage and extramedullary disease. She did not carry other risk factors.
The patient was initially treated with thalidomide + dexamethasone for 2 cycles only due to intolerance to toxicities. She was then switched to melphalan + prednisone which was stopped after 4 cycles due to inadequate responses. After changing to the regimen of bortezomib-based regimen, the condition of the patient was stabilized, and a PR was achieved after 8 cycles of treatment. The patient experienced grade 2 PN during the treatment course, which resolved to grade 1 after completing the 8 cycles of bortezomib-based therapy.
During a regular visit, progressive disease (biochemical relapse) was noted with the serum paraprotein level increasing to 17g/L about 16 months later. In this patient, the treatment goal was to maximize treatment response and the duration of responses. After discussing with the patient, she favored oral treatment since she hoped to maintain her high QoL with less frequent hospital visits. Therefore, IRd was started in September 2016.
The patient was monitored monthly for treatment compliance and tolerance, changes in serum paraprotein level and end-organ damages. Notably, the response of these patients to IRd was rapid, with the serum paraprotein level dropping from 17g/L to 6.8g/L. The PR was maintained for >5 years. The treatment was switched again only in May 2022 to another oral regimen of pomalidomide-based regimen due to the gradual biochemical relapse.
Throughout the treatment course, the patient only experienced grade 1 diarrhea which was effectively managed with loperamide. Her high QoL was well maintained. Overall, the patient was very satisfied with the treatment responses and minimal AEs of IRd.
Discussion
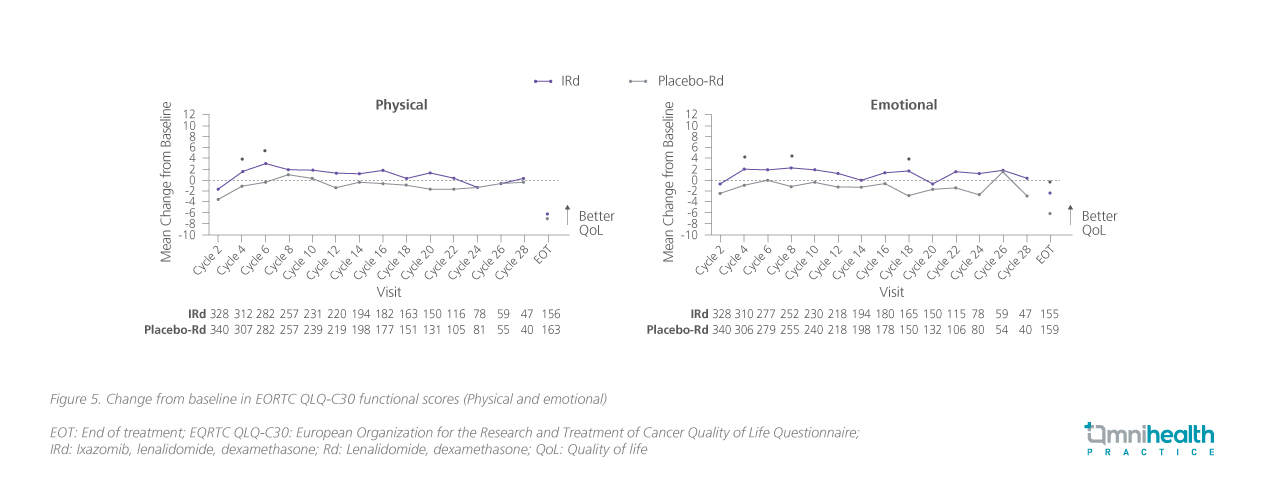
MM has a substantial negative impact on QoL among MM patients, reducing their physical and emotional functioning.4 A prespecified secondary analysis of the TOURMALINE-MM1 study found that for patients treated with IRd, the patient-reported HRQoL was well maintained throughout the study, in addition to the improved efficacy vs. Rd alone.4 Specifically, mean European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EQRTC QLQ-C30) global health status (GHS)/QoL scores were maintained from baseline over the course of treatment in both groups, with no statistically significant differences between groups throughout the study (figure 5, physical and emotional only).4 “Since the MM diagnosis in 2013, this patient has been staying with the disease for almost 10 years now,” Dr. Wong said. During these 10 years, the patient was able to achieve adequate disease control while maintaining normal daily activities and high QoL. Overall, the patient was satisfied with the treatment outcomes.
Take-home messages
Dr. Wong emphasized that nowadays MM patients can enjoy a generally long-term survival thanks to the therapeutic advance. Clinicians should not solely aim to achieve deep treatment response in RRMM patients. Instead, attention shall also be paid to patients’ QoL and their preferences. “Since the ixazomib-based oral regimen can benefit most patients with MM and help them stay in treatment and maintain their high QoL, it can be a good treatment option for those who particularly value convenience and QoL,” Dr. Wong stated.
"Since the ixazomib-based oral regimen can benefit most patients with MM and help them stay in treatment and maintain their high QoL, it can be a good treatment option for those who particularly value convenience and QoL."
Dr. Wong, Siu-Ming Raymond
Head of Division, Division of Hematology,
Department of Medicine & Therapeutics,
Faculty of Medicine,
The Chinese University of Hong Kong
Conclusion
In summary, although MM remains incurable, the recent advance in this therapeutic area has allowed most patients to enjoy a very long-term survival. The all-oral IRd regimen has demonstrated rapid and durable responses, and favorable long-term safety among patients with RRMM. Due to the prolonged survival, most hematologists now consider patients’ preferences and QoL important in the management of RRMM. IRd provides a convenient, well-tolerated and effective option for the RRMM patients, helping them stay in treatment and decreasing their need for frequent hospital visits. With the benefits of maintaining patients’ high QoL, IRd can personalize RRMM management and better meet the patient’s needs.

