MEETING HIGHLIGHT
An equally efficacious and safe alternative: The once-weekly carfilzomib-based therapy
Multiple myeloma (MM) is an incurable hematological disease with a global incidence of 160,000 cases in 2018.1,2 Since treatment failure among patients with relapsed/ refractory multiple myeloma (rrMM) was common with conventional therapies, additional treatment options are urgently needed to improve the survivals of these patients.2,3 At the recently held Annual Scientific Meeting of Hong Kong Society of Myeloma, Dr. Keith Stewart revealed the merits of carfilzomib, a second-generation proteasome inhibitor, as a combination therapy to manage rrMM and highlighted the results of ARROW - the first randomized phase 3 trial comparing the efficacy and safety of twice-weekly carfilzomib + dexamethasone (Kd) regimen with the once-weekly Kd regimen.
Prevailing treatment regimens and their limitations
The POLLUX trial demonstrated that the combination of daratumumab with lenalidomide and dexamethasone (DRd) was more effective than lenalidomide and dexamethasone (Rd) in the first or second relapse.4 When compared with the Rd group, the progression-free survival (PFS) was significantly prolonged in the DRd group (17.5 vs. 44.5 months; HR=0.44; 95% CI: 0.35-0.55; p<0.0001).5 “This combination of drugs is a compelling regimen for use in the early treatment course. However, the drawback is that both treatment regimens depend on the sensitivity of patients to lenalidomide,” Dr. Stewart stated. Unfortunately, the use of lenalidomide for MM management is common and is typically continued until disease progression or intolerance. Most patients have become refractory to lenalidomide, and therefore, these regimens might not be viable treatment options for bringing survival benefits to this group of patients.3 The findings indicated the need to find better treatment regimens for the lenalidomide-refractory patients.
Novel combination of drugs for rrMM management
Carfilzomib, a second generation proteasome inhibitor, was one of the major advancements in rrMM management, which has been shown to be superior to the first generation proteasome inhibitors, in terms of enhanced efficacy in rrMM treatment, improved safety profile and more convenient administration methods.6 In one of the earlier randomized, phase 3 studies (ASPIRE), the efficacy, safety and impact on health-related quality of life (HRQoL) of carfilzomib were evaluated in rrMM patients who were randomized to receive either carfilzomib with lenalidomide and dexamethasone (KRd), or lenalidomide with dexamethasone (Rd).7 Due to certain unknown long-term toxicities and patients’ concerns, carfilzomib was discontinued after 18 cycles of use 7,8. In spite of this, the results still showed a significantly prolonged PFS with the addition of carfilzomib when compared with control group (26.3 vs. 17.6 months; (HR=0.69; 95% CI: 0.57-0.83; p=0.001).8 In addition, a higher proportion of patients with carfilzomib treatment met the global health status/ quality of life (GHS/QoL) criteria at cycle 12 (25.5% vs. 17.4%) and cycle 18 (24.2% vs. 12.9%) compared with the Rd group.7
ENDEAVOR: A pivotal study on the efficacy of carfilzomib or bortezomib in rrMM
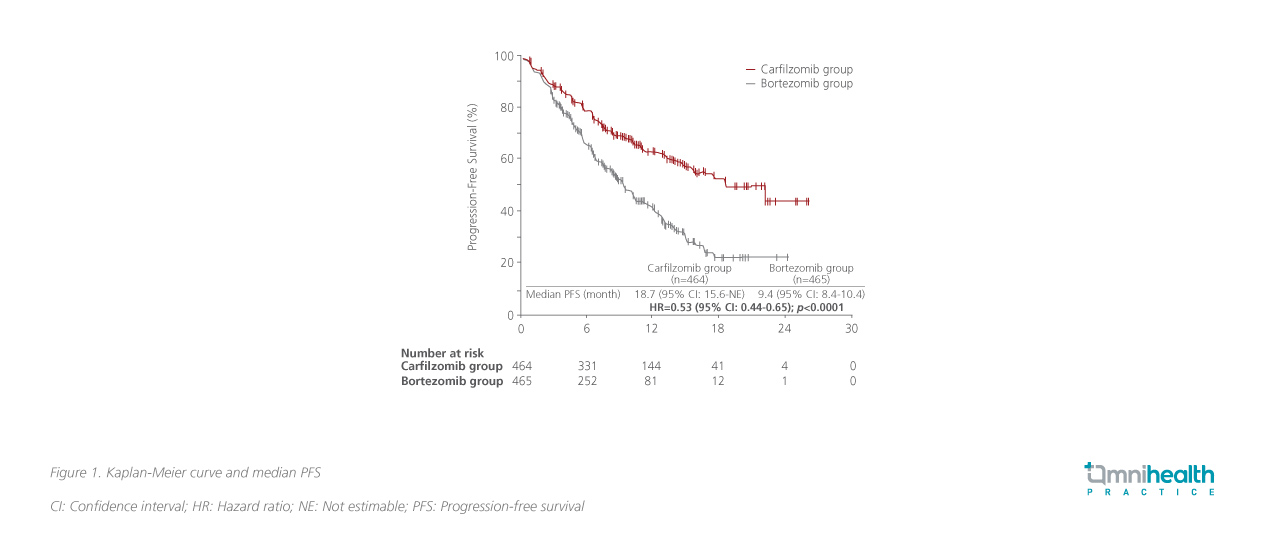
ENDEAVOR, a phase 3 trial, was conducted in rrMM patients to compare the clinical efficacy of carfilzomib and bortezomib head to head.9 Both agents are inhibitors of the intracellular proteasome pathway, with carfilzomib being structurally different from the first generation bortezomib for its increased selectivity and potency.10 This study randomized 929 patients with rrMM to bortezomib-dexamethasone (the bortezomib group) vs. carfilzomib-dexamethasone (the carfilzomib group).10 Based on the results, the median PFS was observed to be 18.7 months in the carfilzomib group and 9.4 months in the bortezomib group (HR=0.53; 95% CI: 0.44-0.65; p<0.0001) (figure 1).9 Similarly, the response rate was significantly higher in the carfilzomib group compared with the bortezomib group (77% vs. 63%).9
ARROW: A study aims to optimize dosing schedule of carfilzomib in rrMM
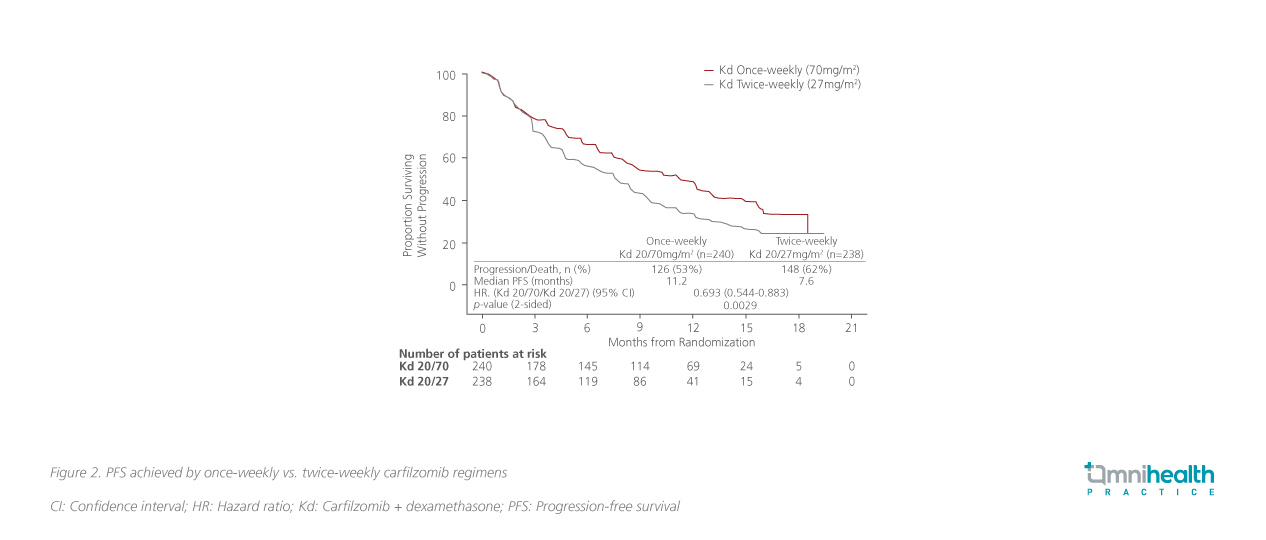
ARROW is a randomized, open-label, phase 3 trial conducted with rrMM patients who received 2-3 prior lines of therapy.11 The patients were eligible to receive therapy based on prior bortezomib or ixazomib treatment and were aged 18 years and above. In this study, 478 patients were equally assigned to receive carfilzomib once a week (20/70mg/m2) or twice a week (20/27mg/m2). Both groups received dexamethasone 40mg according to the study protocol.11 The once-weekly group was intravenously infused for 30 minutes with carfilzomib on days 1, 8 and 15 of all cycles, with 20mg/m2 day1 (cycle 1) and 70mg/m2 thereafter. On the other hand, the twice-weekly group was intravenously infused for 10 minutes with carfilzomib on days 1, 2, 8, 9, 15 and 16 with 20mg/m2 on days 1 and 2 during cycle 1 and 27mg/m2 thereafter.11
The overall response rate (ORR) was higher in the once-weekly group as opposed to the twice-weekly group (63% vs. 41%). Moreover, the OS and safety were comparable between the 2 groups. Treatment-related adverse events of interest were also similar between both groups. As shown in figure 2, once-weekly carfilzomib at 70mg/m significantly prolonged PFS compared with the twice-weekly schedule at 27mg/m2 (11.2 vs. 7.6 months; HR=0.69; 95% CI:0.54- 0.88; p=0.0029).11 “Once-weekly dosing of carfilzomib appears to be a safe, convenient and effective dosing option for rrMM treatment,” Dr. Stewart emphasized. It is suggested that the once-daily dosing schedule is a viable treatment alternative to the twice-week dosing regimen, which can be adopted for improving patient adherence and tolerance to treatment.11 However, it should be noted that the twice-weekly regimen had also been shown to achieve a deep and durable response among rrMM patients in the ENDEAVOR and ASPIRE studies. Therefore, the latter should still be considered as a suitable treatment option for achieving good outcomes for patients who are tolearable.8,9
The twice-weekly (20/27mg/m2) regimen was adopted in the ARROW trial since it was the only approved dosing when ARROW was conducted. However, this regimen has been less commonly used nowadays and no longer approved dosing for carfilzomib. As such, a post-hoc analysis was performed to compare the safety and efficacy of once-weekly (20/70mg/m2) regimen with the commonly used twice-weekly (20/56mg/m2) regimen for a fairer comparison.
Post-hoc analysis: Once-weekly (70mg/m2) vs. twice weekly (56mg/m2) dosing of carfilzomib in rrMM patients
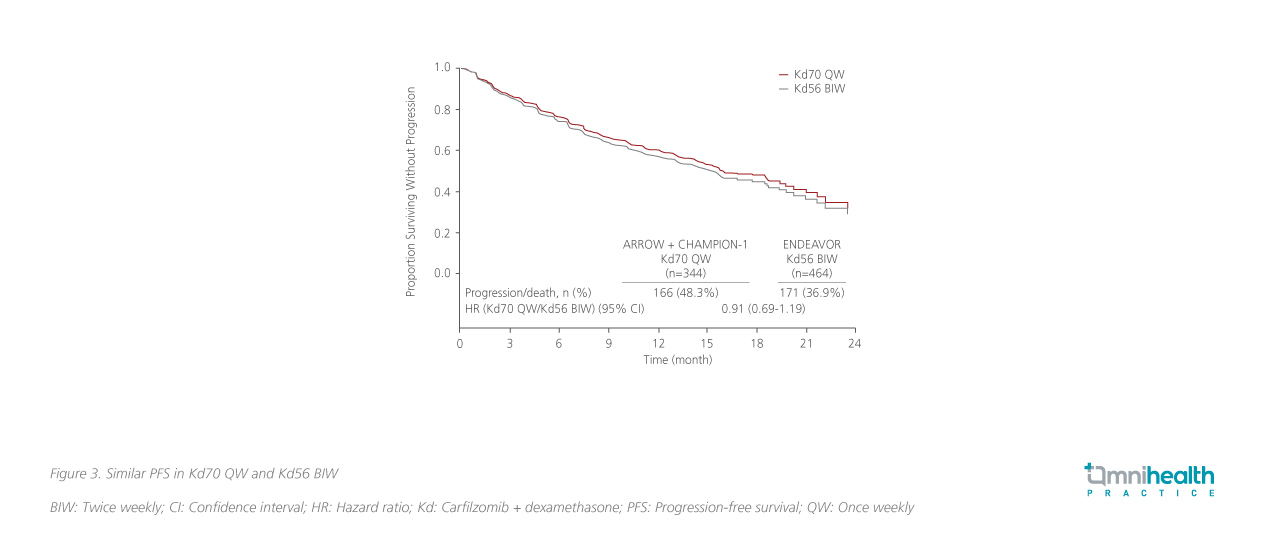
The post-hoc cross-trial comparisons, which included ARROW, CHAMPION-1 and ENDEAVOR, were performed to compare the efficacy and safety profiles of once-weekly 70mg/m2 Kd dosing (Kd70) with the more commonly used twice-weekly 56mg/m2 Kd dosing (Kd56) (figure 3).12 The side-by-side efficacy and safety comparisons were done in the subgroups of patients with 2-3 prior lines of therapy, who were not refractory to bortezomib. Some 146 patients who received once-weekly Kd70 in ARROW and CHAMPION-1 were compared with the patients (N=217) who received Kd56 twice-weekly in ENDEAVOR. The results showed that their efficacies were similar between once-weekly Kd70 and twice weekly Kd56, with the ORR being 69.9% vs. 72.4%, respectively.12 Their achieved median PFS was also comparable (12.1 months vs. 14.5 months).12 There was no significant difference between the 2 groups after adjusting for prognostic covariates. These results revealed that their efficacy and safety profiles were similar.12
CV toxicity management in rrMM
Despite the promising data substantiating carfilzomib as an essential drug to rrMM treatment, many clinicians were concerned about the cardiovascular (CV) toxicities arising from the carfilzomib treatment, especially among those patients with pre-existing CV diseases.13 Dr. Stewart stressed that preemptive assessment by cardiologists before treatment initiation and regular monitoring would suffice for patients experiencing ischemic heart disease or arrhythmia. However, for patients with severe hypertension, an initial lower dose of 20mg/m2-27mg/m2, followed by a gradual dose escalation based on the treatment response, would be more appropriate. In addition, the measurement of CV marker, i.e. levels of N-terminal B-type natriuretic peptide (NT-proBNP), should be considered as it would be informative for patients with cardiac problems.14
Conclusion
The aforementioned clinical studies clearly indicated that carfilzomib is an effective drug for rrMM patients with a manageable safety profile. Previously, the twice-weekly Kd56 regimen was a more commonly used treatment, but it was possibly associated with poor treatment adherence and lower patient tolerability. The recent post-hoc analysis has revealed that the more convenient once-weekly Kd70 demonstrated a comparable efficacy and safety profile to the twice-weekly Kd56, and hence that can be considered as an alternative for rrMM patients with adherence or tolerability issues. However, for patients without such problems, the twice-weekly Kd56 remains an important treatment option for achieving deeper and durable responses. All in all, these newly available data highlighted the clinical efficacy and safety of the combination therapies using carfilzomib for rrMM management, which are available in either once-weekly or twice-weekly dosing schedule, catering to the needs of different rrMM patients.

