MEETING HIGHLIGHT
Real-world analysis found ixazomib triple therapy effective in the management of relapsed/refractory multiple myeloma
Although the median overall survival (OS) of patients with multiple myeloma (MM) has significantly improved over the past two decades, MM remains an incurable malignancy with most patients experiencing relapse and requiring additional therapy.1 Among these patients, the presence of clonal heterogenicity may lead to the difference in response to treatment and ultimately to relapsed and refractory status.1 Recently, the TOURMALINE-MM1 trial has demonstrated that the combined ixazomib, lenalidomide and dexamethasone (IRd) regimen is an effective treatment for relapsed/refractory MM (rrMM).2 Most significantly, these findings have shown excellent translation into the real-world setting.3 In a recent symposium, Professor Graham Jackson, Freeman Hospital, The Newcastle Upon Tyne Hospitals, NHS Foundation Trust, United Kingdom, discussed the role of IRd triple therapy in the management of rrMM in the real-world setting.
Response to rrMM treatment is highly heterogeneous
MM is a complex disease, characterized by inter-patient and intra-tumor heterogeneity.4 The usual treatment of rrMM is often accompanied with periods of remissions and relapses. Eventually, treatment for relapses are no longer successful and the disease becomes refractory. According to an European database on the proportion of patients reaching each line of therapy, among the newly diagnosed MM patients, around 95% made it into the first-line of therapy and 61% into the second-line; in MM patients receiving first- and second-line therapy, only 38% and 15%, respectively, reached subsequent lines of therapy.5 When first diagnosed of MM, the median duration of first-line therapy for these patients was 6 months, followed by a median treatment-free interval of 10 months.5 With increasing lines of therapy after relapsed/refractory disease, both these durations, the time to progression and the depth of response began to decrease.5
The prognosis of rrMM closely follows the genomic composition of the patient population with approximately one-third of patients with MM having stable genomes, thus a lower risk and more favorable clinical outcomes.1 In the other two-thirds, their genome are less stable and are more prone to changes over time due to clonal heterogeneity and linear evolution.1 Furthermore, Prof. Jackson mentioned that the prognosis of rrMM is also dependent on the stage and clinical presentation of the disease, frailty, comorbidities, response to standard treatment and timing of first relapse.
Identifying the appropriate treatment to rrMM
According to the ESMO 2017 guidelines, a variety of dual or triplet therapies with dexamethasone and bortezomib (Rd) as backbone and the addition of ixazomib (IRd), lenalidomide (VRd), panobinostat (PanoVD), carfilzomib (KRd), daratumumab (DaraRd), or elotuzumab (EloRd) are commonly used for treating rrMM.6 Notably, IRd triplet therapy has demonstrated significantly longer PFS and a positive benefit-risk ratio with meta-analysis placing IRd as one of the highest performing triplet therapies.2,7,8 “When considering the treatment response, the triplet of IRd has a clinical benefit over duplet therapy,” stated Prof. Jackson. Additionally, a pooled analysis by Palumbo et al. has demonstrated a significantly improved PFS of 32 versus 16 months and 4-year OS of 69% versus 60% when MM patients were prescribed with continuous therapy over fixed therapy.9 By applying these results to the rrMM setting, a continuous triplet therapy should be considered when treating patients with rrMM.
That said, four different groups of factors, including patient factors, disease burden, prior therapy and socioeconomic hurdles, should also be considered before determining the most appropriate type of rrMM treatment and duration.10,11 With IRd being capable of achieving favorable long-term outcomes balanced with manageable toxicity and maintenance of quality of life, IRd should be considered a candidate for continuous triplet therapy for rrMM treatment.9,12,13
TOURMALINE-MM1: Translating clinical observations into the real-world
While previous clinical studies suggested the benefits of various treatment options in rrMM treatment, many of them may not reflect the real-world MM population especially when poorer long-term outcomes were observed in the real-world than in the clinical trials. Particularly, the ranges of median PFS or time to next therapy (TTNT) in previous real-world reports were found to be shorter than those reported in phase 3 clinical trials evaluating regimens used in routine clinical rrMM treatment.14 In the real-world setting, around 40% of MM patients treated in community or academic institutions are not eligible for clinical trials.15 Among these ineligible patients, a lower 3-year OS can be observed when compared to those who are eligible for clinical trials.15 Moreover, specific patient populations, such as elderly or frail patients and those with comorbidities, advanced disease or aggressive and rapidly progressing disease, as well as some ethnic or racial minorities, are typically under-represented in clinical trials.16
To evaluate the effectiveness of the oral protease inhibitor (PI) immunomodulatory drug triplet regimen of IRd, the TOURMALINE-MM1 trial was conducted with intention to reflect the real-world use of IRd. Accordingly, the TOURMALINE-MM1 is a double-blind, placebo-controlled, phase 3 trial where patients with rrMM were randomized to receive either IRd or placebo plus lenalidomide-dexamethasone.2 Based on the results, the median PFS was observed to be 20.6 months in the ixazomib group and 14.7 months in the placebo group (HR= 0.74; 95% CI: 0.59-0.94; p=0.01), representing a 35% longer PFS with ixazomib regimen.2 The benefit with respect to PFS was consistent in all key prespecified patient subgroups, including patients with a poor prognosis such as those with high-risk cytogenetic abnormalities, those with International Staging System stage III disease, those older than 75 years of age and those who had received two or three prior therapies.2 With regard to prior therapies, PFS benefit with ixazomib appeared greater in patients with 2 or 3 prior lines of therapy when compared to the placebo group.17
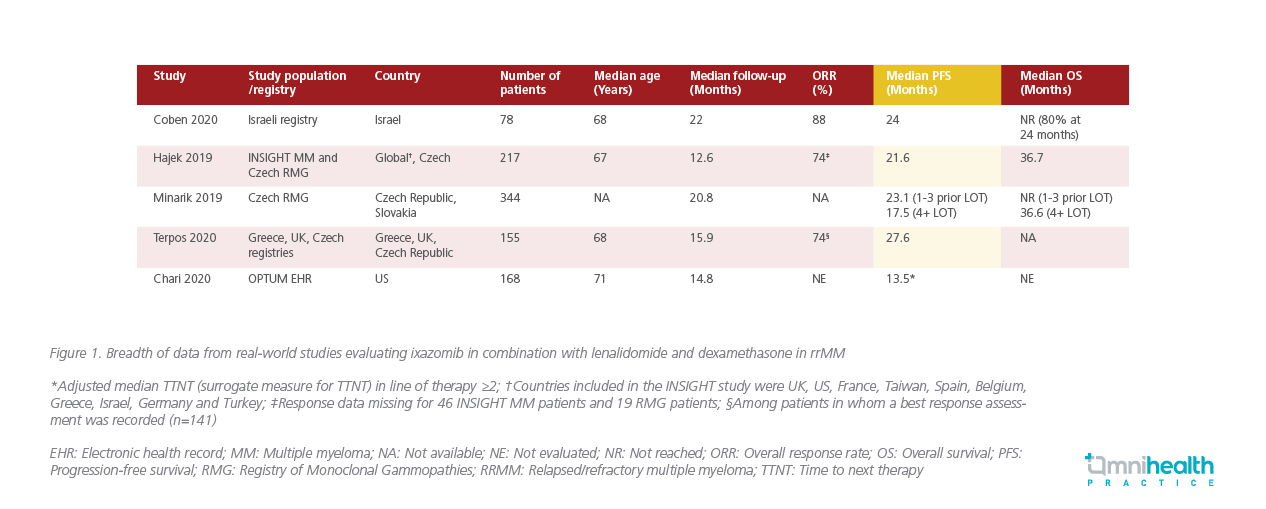
When compared to the real-world setting, the PFS benefit of IRd observed in the TOURMALINE-MM1 trial was comparable to multiple real-world studies that evaluated the efficacy of IRd in the rrMM setting (Figure 1). According to the real-world multi-site Israeli registry study, ixazomib-based combinations were efficacious and safe in treating rrMM patients regardless of the cytogenetic risk, with a PFS of 24 months comparable to the TOURMALINE-MM1 trial.16 Similarly, Hajek 2019 et al. found that the effectiveness of IRd in routine clinical practice was comparable to the efficacy of IRd reported in the TOURMALINE-MM1 trial.18 In addition to having a well-tolerated safety profile with no new safety signals observed, a lower rate of dose reductions due to adverse effects was observed with the real-world use of IRd.18 Moreover, in an analysis of the Czech registry data, the oral IRd regimen was found to have improved PFS with long-term benefits comparable to the TOURMALINE-MM1 trial.19 In another multi-centric, retrospective analysis, IRd was demonstrated to be effective and well-tolerated in the real-world clinical setting.20 Based on the similarities in efficacy and safety between real-world data and the TOURMALINE-MM1 trial, Prof. Jackson emphasized that, “We know that IRd does well in the clinical trial settings, it is deliverable and achieves the same responses and same PFS in community-based assessment of response [when compared to the TOURMALINE-MM1 trial].”
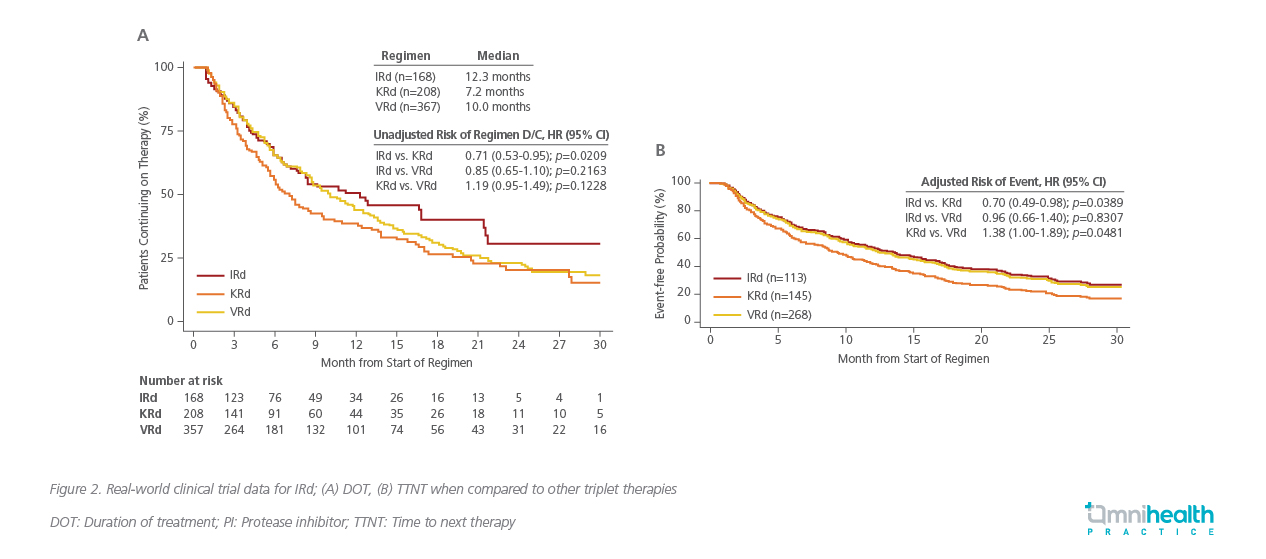
Compared to other triplet regimen such as KRd (carfilzomib, lenalidomide, dexamethasone) or VRd, the median duration of therapy (DOT) for the IRd regimen was shown to be comparatively longer at 12.3 months versus 10 months for VRd and 7.2 months for KRd (Figure 2A).21 Additionally, among those with intermediate risk such as frail patients, IRd was also associated with a longer TTNT when compared to other triplet regimen (Figure 2B).21 Prof. Jackson reiterated, “Evidence supports IRd as a probably easier to deliver regimen in the clinical setting for longer durations.”
IRd is effective regardless of underlying cytogenetics risks
While a large portion of rrMM patients have high-risk cytogenetics that are more prone to unfavorable clinical outcomes, IRd has demonstrated a similar median PFS benefit of 21.4 months among those with high-risk cytogenetic features when compared to 20.6 months among those with standard-risk cytogenetic features.2 When compared to patients in the placebo arm of the TOURMALINE-MM1 trial, patients in the ixazomib arm demonstrated an improved PFS of 11.7 months (21.4 vs. 9.7 months, HR=0.54; 95% CI: 0.32-0.92; p=0.02).2 Even in those with del(17p) or t(4;14) without del(17p) and t(14;16) aberrations, a PFS benefit of 21.4 and 18.5 months, respectively, were observed with IRd.2
As the only oral triplet treatment, IRd treatment was reported to have maintained a similar health-related quality of life from baseline over the course of treatment when compared to the control arm in the TOURMALINE-MM1 study.12 Similarly, the physical, emotional and social function domains were also maintained with IRd treatment.12 “IRd triplet therapy is recommended for patients with high-risk cytogenetics, and when we look particularly at the TOURMALINE MM1 trial data, when compared to the standard risk, the high-risk group has completely overcome the risk barrier,” concluded Prof. Jackson.
Conclusion
The treatment landscape in rrMM is constantly evolving with an increasing range of therapeutic options now being added to the intricate clinical treatment decision-making process. Careful interpretation of the clinical trial data is vital and must be merged with real-world considerations. With the efficacy and safety of the IRd triplet regimen observed in the TOURMALINE-MM1 trial being comparable to the real-world setting, clinicians should now consider IRd as an alternative option for the treatment of rrMM patients.

