MEETING HIGHLIGHT
Promising real-world evidence of all-oral triplet combination of ixazomib, lenalidomide, and dexamethasone (IRd) for the treatment of rrMM
Although the median survival rate in multiple myeloma (MM) patients has doubled in recent years, it remains incurable as most patients relapse and become refractory to drugs, requiring alternative therapies that involve novel agents.1,2 At the Annual Scientific Meeting of Hong Kong Society of Myeloma, Professor Heinz Ludwig, Director of Wilhelminen Cancer Research Institute, Austria presented the real-world evidence (RWE) of ixazomib, lenalidomide and dexamethasone (IRd) in managing refractory/relapsed multiple myeloma (rrMM) and showed that the real-word data confirm the positive results as seen in previous clinical trials of IRd in treating rrMM. Prof. Ludwig stressed the need to translate these promising RWD on the effectiveness of IRd regimen into clinical practice when making decisions to treat rrMM patients.
Challenges in treating patients with rrMM
Approximately one-third of the MM patients are standard-risk patients, where their genomes show few changes over time and they respond well to medication. The remaining two-thirds are intermediate or high-risk patients. In this group of patients, clonal heterogeneity and evolution and accumulation of mutations in individual genes make them more likely to become refractory to previously-used treatment.3 Thus, combination therapies which target multiple pathways may be more effective. Traditionally, therapies comprised of bortezomib/dexamethasone (VD) backbone and combined with lenalidomide (VRd) or cyclophosphamide (VCd) have been used in the treatment of rrMM.4,5 The addition of novel agents such as proteasome inhibitors (PI), histone deacetylase inhibitors, immunomodulators, and monoclonal antibodies to the regimen has shown promising results in several RCTs and demonstrated superior clinical outcomes compared to the traditional doublet therapy.5 Ixazomib is the first oral PI to be approved by the US Food and Drug Administration (FDA) in 2015 for the treatment of MM in combination with lenalidomide and dexamethasone based on the seminal TOURMALINE MM1 clinical trial.
The seminal TOURMALINE MM1 study on the efficacy of ixazomib triple therapy
TOURMALINE MM1 is a double-blind, placebo-controlled, phase 3 trial that randomized 722 patients with relapsed, refractory, or relapsed and refractory MM to receive either the IRd regimen or the placebo-Rd regimen.6 The primary endpoint of the study was progression-free survival (PFS). Baseline characteristics of the patients were well balanced between groups, but Prof. Ludwig brought attention to the fact that almost 70% of the patients were exposed to PIs previously, which he said “was quite remarkable”.
The TOURMALINE MM1 study showed that in relapsed, refractory, or relapsed and refractory MM patients, treatment with the IRd regimen resulted in a significant improvement of PFS as compared to placebo-Rd (20.6 vs. 14.7 months; HR=0.74; 95% CI: 0.59-0.94, p=0.01).6 Additionally, the benefit of the IRd regimen extended to subgroups of patients with a poor prognosis, including elderly patients, patients who have received two or three prior therapies, patients with advanced-stage disease and patients with high-risk cytogenetic abnormalities. Prof. Ludwig noted that ixazomib seemed to abrogate the negative impact of high-risk cytogenetics on PFS as there was no difference in PFS in the high-risk [patients with del(17p)] versus standard-risk [patients with t(4;14) alone] population. Response rates to IRd were rapid, durable and deepened with increasing duration to the treatment, and the number of responders showing complete response (CR) was higher in the IRd group versus placebo-Rd.6 In terms of safety, IRd was well-tolerated with only limited additional toxicity.6
China Continuation Study (CCS) confirms the efficacy of adding ixazomib to Rd
The CCS was an expansion of the TOURMALINE-MM1 study consisting of 115 patients.7 Prof. Ludwig brought attention to the fact that there were three key differences in the study population of the CCS. It included patients that relapsed earlier, it had more relapsed and refractory patients, and more patients in the CCS were exposed to an IMiD, namely thalidomide and were thalidomide-refractory.7 Despite this, the overall response rate (ORR), very good partial response (VGPR), and CR were significantly better in the IRd group versus the placebo-Rd group.7 Furthermore, a significant improvement of 67% in PFS (6.7 vs. 4.0 months; HR=0.598; 95 CI: 0.367-0.972, p=0.035) as well as a 138% improvement in overall survival (OS) (25.8 months vs. 15.8 months; HR=0.419; 95% CI: 0.242-0.726, p=0.001) were observed in the IRd group versus placebo-Rd group, which was not seen in the TOURMALINE MM1 study.7 Similar to the TOURMALINE MM1 study, there was limited additional toxicity as compared to placebo-Rd; moreover, the incidence of anemia was less in the IRd group.7
Comprehensive understanding of IRd via real-world evidence (RWE)
Although both TOURMALINE MM1 and CCS showed promising results for IRd, RCTs have inherent limitations for clinicians to translate these results into clinical practice. RCTs usually have strict exclusion and inclusion criteria to enroll patients and often do not reflect the entire population. Moreover, real-world conditions differ considerably from a clinical trial set-up. For example, age of patients presenting with rrMM in clinics is usually higher, there are more patients with urgent need of treatment initiation, who cannot await the usually lengthy screening process needed for trial enrollment, and there are more patients present with comorbidities in real-world conditions. Real-world PFS and time-to-next treatment (TNTT) values have been shown to be shorter than those seen in rrMM RCTs.8 According to a study, strict enrollment criteria in RCTs made almost 72% of rrMM patients in clinical practice ineligible for the pivotal studies that were used to approve drugs for rrMM.9 Additionally, racial minorities are usually underrepresented in RCTs. Therefore, real-world data obtained through routine clinical practice must be considered along with RCT results as RWE could fill the gaps between the efficacy of an intervention in controlled trial settings and the outcome in treating a heterogeneous population of patients in clinics worldwide. Prof. Ludwig presented the RWE analyzed from four independent studies that prescribed IRd to rrMM patients who had at least one prior line of therapy. The results confirmed the findings observed in the RCTs, especially with respect to median PFS (Figure 1). He emphasized that IRd should be considered a treatment of choice as it was demonstrated to be efficacious in both RCTs and the real-world clinical setting.
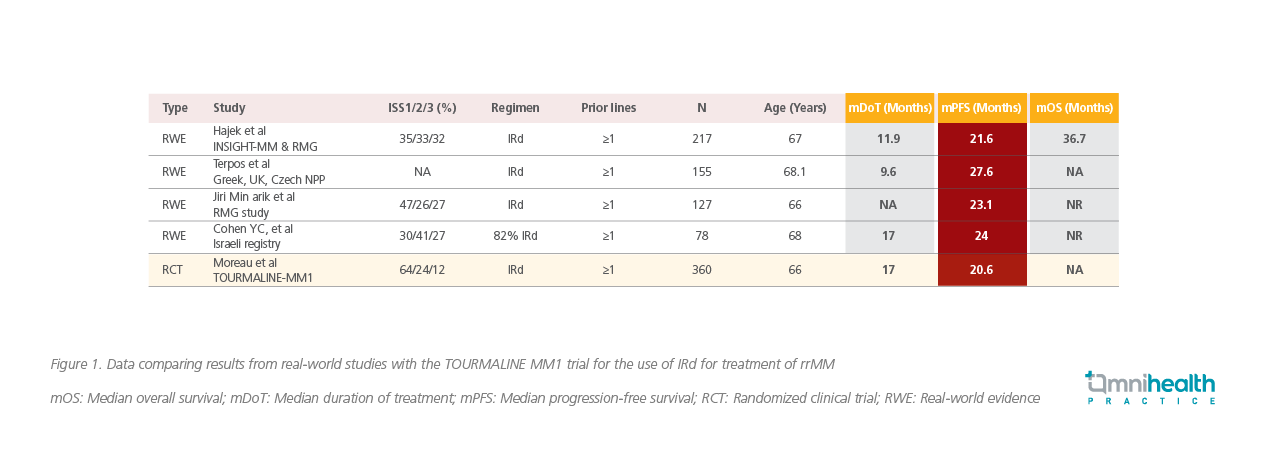
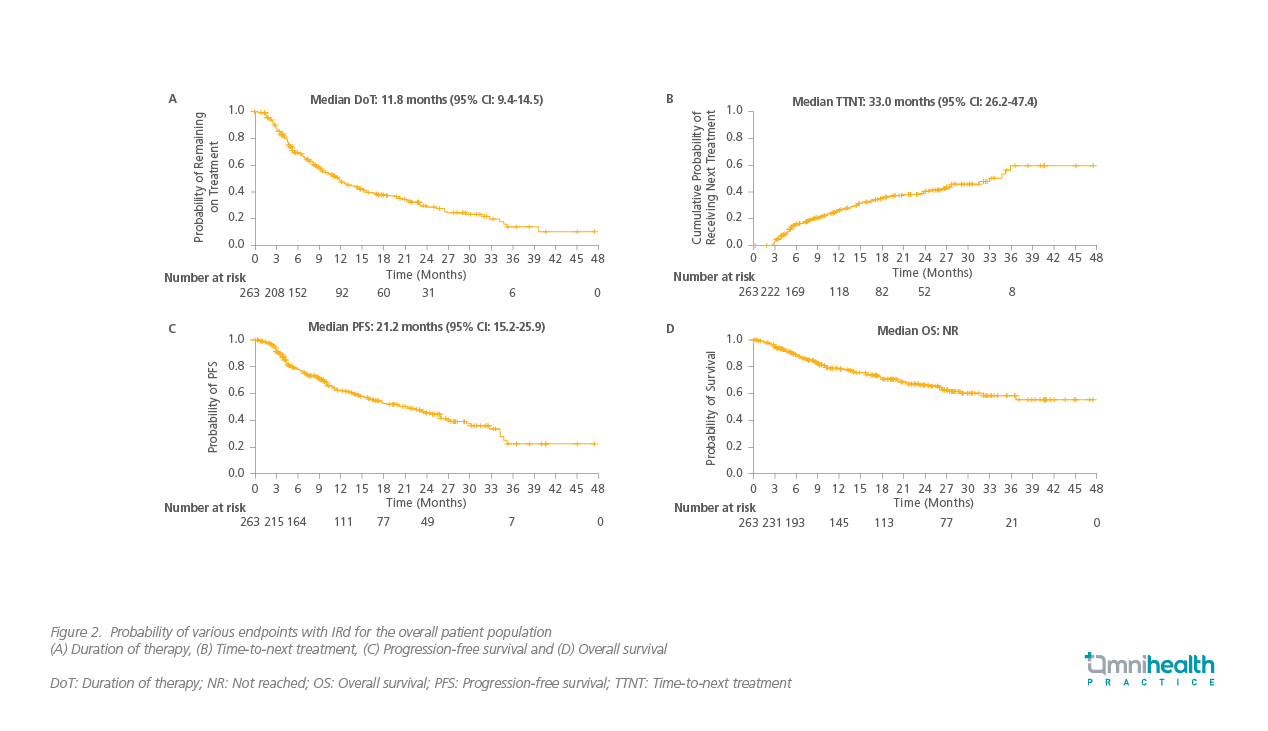
Hajek et al. conducted a prospective observational study, which was an extended pooled analysis of clinical data gathered from the Czech Registry of Monoclonal Gammopathies (RMG) and the INSIGHT MM study.10 They evaluated PFS, ORR, TTNT, and duration of treatment (DoT), apart from safety profile of MM patients on IRd regimen.10 In the 217 included patients, Dr. Ludwig noted that “ORR was remarkably high (73%) for patients who took IRd therapy”. These patients had exceedingly well TNTT and longer median PFS and DoT (Figure 2).11 IRd was well-tolerated with no new safety signals.11 The study concluded that the overall results were comparable to the TOURMALINE MM1 clinical trial, despite the fact that the patient population in this study had presented with more advanced stage of MM.11
On the other hand, Terpos et al. conducted a multi-center, retrospective, observational analysis of real-world data pooled from the Czech, UK, and Greece databases of MM patients treated with IRd (N=155).12 The patients in this analysis had more adverse characteristics and advanced disease. Patients had received more prior lines of therapy within a shorter period since diagnosis of their MM and 97% patients had received prior treatment with a PI.12 The median PFS was 27.6 months in patients receiving IRd as second-line therapy.12 There was no significant difference in PFS in patients receiving IRd who had prior exposure to other therapies or transplants, except for patients who were exposed to lenalidomide – they had a lower PFS.12 ORR was very good (74%). Here as well, the researchers found that patients from this analysis had comparable results and similar safety profile to the TOURMALINE MM1 study.12
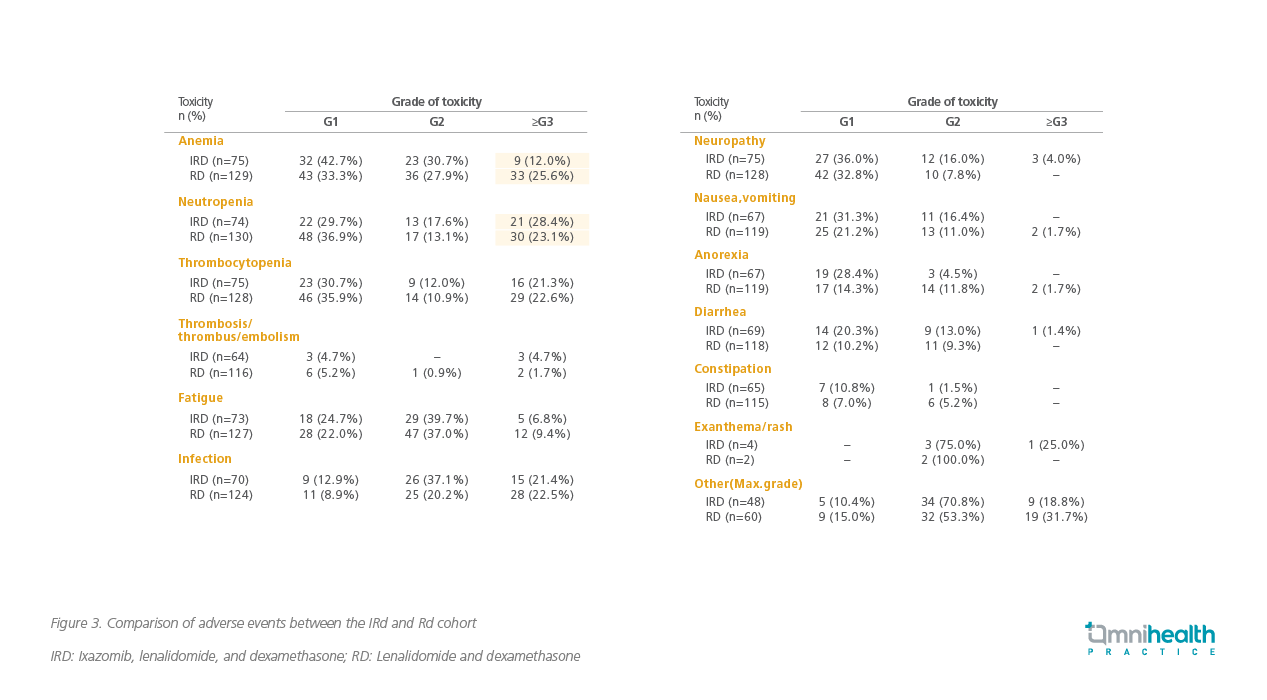
Another retrospective comparative cohort analysis of real-world data from RMG by Minarik et al. analyzed data from 344 rrMM patients, where 127 patients received IRd and 271 patients received Rd.13 Patients with 1-3 relapses had significantly longer PFS with the IRd regimen (23.1 months) versus those who were on Rd regimen (11.6 months).13 All subgroups benefitted from IRd. OS was significantly better in all patients in the IRd cohort than the Rd cohort (36.6 months vs 26.0 months), and OS was not reached in the IRd group in those who had 1-3 lines of prior therapy.13 Response rates were also higher, especially in the VGPR, in the IRd group. PFS and OS in groups with or without prior transplant were better in the IRd group; except for patients who had an extramedullary disease, who did not do as well with the IRd regimen.13 Anemia was found to be less pronounced and neutropenia was found to be more frequent in the IRd arm vs. Rd alone, otherwise similar adverse events were observed in both the groups (Figure 3).13 This study demonstrated the OS benefit of IRd over Rd in clinical practice and confirmed that the long-term outcome of IRd regimen in real-world is comparable to what was observed in the TOURMALINE MM1 RCT.13
In a smaller study (N=78), RWD from the Israel patient registry on ixazomib-based combinations for rrMM analyzed by Cohen et al. showed that patients achieved an ORR of 88%, at 2nd as well as later lines of therapy regardless of the cytogenetic risk.14 Patients had median PFS of 24 months over a 2-year follow-up period.14 The study found that ixazomib-based treatment was well-tolerated and had lower discontinuation rate, concluding that these combinations are efficacious and safe in rrMM patients in the real-world setting.14
RWD for IRd regimen was also compared to other triplet regimens involving Rd in a study from the United States. A retrospective, comparative analysis of TNTT in 733 rrMM patients who initiated a triplet regimen containing ixazomib (I), carfilzomib (K), or carfilzomib (V) plus an Rd backbone (i.e. IRd, KRd, or VRd respectively) in routine clinical practice was conducted by Chari et al.15 The study found that intermediate-to-frail patients had longer TNTT in the IRd and VRd group vs. KRd. Not only that, patients with higher cytogenetic risk or prior exposure to immunomodulatory drugs did better with IRd as compared to VRd.15
Another retrospective analysis of real-world data (N=820) took the same database as above and compared the above-mentioned regimen (IRd, VRd, and KRd) to daratumumab-Rd (DRd).5 Here also, IRd showed similar results when compared to VRd, but DRd patients did significantly better in event-free survival as compared to the rest of the triplet therapies.5
Prof. Ludwig concluded that, “In my opinion, ixazomib-Rd treatment for relapsed refractory multiple myeloma offers several benefits; it’s an oral treatment, it is effective even in cytogenetic high-risk patients, it is well-tolerated, so I think in our hands it is a frequently used regimen in elderly patients, patients who are not candidates for transplant, or have had prior exposure to transplant do quite well.”
Conclusion
Outcomes in rrMM patients treated with IRd in clinical practice are comparable to results observed in clinical trials, as confirmed by many studies analyzing RWD. IRd regimen was found to be efficacious and well-tolerated even in elderly and cytogenetically high-risk patients. Moreover, IRd regimen is oral, so it is the most convenient mode of administration. To conclude, Ixazomib triple therapy is a viable treatment of choice for rrMM patients.

