CASE REVIEW
The real-world use of ixazomib achieved stable disease for nearly 3 years, longer than in clinical trial
Multiple myeloma (MM) is the second most common hematological malignancy and is associated with significant morbidity due to its end-organ destruction.1 Despite significant treatment advances in the past couple decades, MM remains an incurable malignancy with most patients eventually experience relapses that require additional therapy.1,2 Previously, the TOURMALINE-MM1 trial demonstrated that the combined regimen of ixazomib plus lenalidomide and dexamethasone (IRd) can significantly improve the progression-free survival (PFS) of relapsed/refractory MM (rrMM) patients.3 In a recent interview with Omnihealth Practice, Dr. Chau Ling Kit, Nicholas, specialist in hematology and hematological oncology, Pamela Youde Nethersole Eastern Hospital, shared a local patient case who had received nearly 3 years of ixazomib-based treatment and had achieved stable disease over the treatment course. Not only was the duration of treatment of this local case longer than the median PFS observed in the TOURMALINE-MM1 trial, the ixazomib-based treatment was also well-tolerated that supports its use in the real-world setting.
Current treatment landscape of rrMM in Hong Kong
From clinical experience, the general prognosis of rrMM in Hong Kong is guarded despite a significant number of patients can achieve reasonable disease control for a considerable period of time. With relapses commonly observed, the increasing incidence of MM has made the disease one of the dominant chronic illnesses in Hong Kong.4 Where transplant-eligible patients can have much improved prognosis with upfront autologous stem cell transplantation (ASCT) and maintenance lenalidomide, it is not uncommon for patients to show a gradual decline in their general well-being with progressive decrease in response duration after multiple lines of therapy.5,6 With cumulative treatments, toxicities including neuropathy, edema, anemia, and overlapping gastrointestinal side effects may also become more prominent.7,8 In the rare exception that transplant-eligible patients choose not to undergo ASCT after initial induction therapy, continuous combination treatment with proteasome inhibitors and immunomodulatory drugs must be maintained to achieve disease control. As the relapse of MM is imminent, the common treatment goal is to control the disease using therapy with acceptable toxicity profile while maintaining the patients’ quality of life.9
A local rrMM case sharing: Real-world use of ixazomib triplet therapy
Previously in the double-blind, placebo-controlled, phase 3 TOURMALINE-MM1 trial, the triplet combination of IRd had significantly improved the median PFS of rrMM patients by 35% when compared to those receiving a plzths; HR=0.74; 95% CI: 0.59-0.94; p=0.01) (Figure 1).3 Notably, the median PFS in the TOURMALINE-MM1 trial did not exceed 2 years, and a similar PFS pattern is generally observed in local practice where second complete remission (CR) is uncommon after the disease has relapsed.3 Despite the poor outlook, real-world studies have demonstrated a translatable PFS benefit of ixazomib-based treatment from the TOURMALINE-1 trial as well as being well-tolerated with a lower rate of dose reduction due to adverse events.10,11 Similarly, a local case presented in 2016 showed that ixazomib-based treatment had achieved disease control while maintaining a good quality of life for the patient in the real-world setting.

In a local rrMM case, a 63-year-old man was presented to the clinic with 2 episodes of severe infection of multilobar pneumonia and hemophilus influenza septicaemia. In September 2016, he was diagnosed of stage 2 immunoglobin G (IgG) kappa myeloma by the International Staging System (ISS). Apart from anemia, other MM features including elevated calcium, renal failure, or bone lesions were not observed. Fluorescence in situ hybridization (FISH) analysis for high-risk cytogenetics was not done as there was no bone marrow examination performed in the local center. Since the patient was on the Comprehensive Social Security Assistance (CSSA) Scheme, his treatment options were limited by the support from the Samaritan funding.
Initially, the patient was treated with first-line bortezomib, thalidomide and dexamethasone (VTD) for 4-cycles as induction to ASCT. However, partial remission (PR) was not achieved with his paraprotein level dropping from 40g/L to 25g/L. Thalidomide was later replaced by cyclophosphamide for 3 more cycles which resulted in stable disease only without further improvement. Despite being eligible for ASCT, the patient decided not to undergo ASCT due to concerns over the treatment risks as well as suboptimal response to the induction therapy.
Additional funding for lenalidomide was later applied and the patient was given 4 cycles of bortezomib, lenalidomide and dexamethasone (VRd) followed by lenalidomide and dexamethasone (Rd) once the funding for bortezomib was exhausted. His paraprotein level then dropped to 15g/dL but did not improve further with continuous lenalidomide treatment. In the first quarter of 2018, he had received IRd treatment until lenalidomide funding was exhausted with cyclophosphamide re-introduced after lenalidomide was stopped.
During the IRd treatment, his paraprotein level fluctuated from 15g/L to 20g/L (Figure 2). Despite not achieving CR, he tolerated treatment well and showed good general well-being. He had experienced mild diarrhea which was not dose limiting. However, early progressive cataracts were observed in 2019 and dexamethasone was stopped. Throughout his ixazomib-based treatment course, he was able to maintain regular table tennis and badminton games with no evidence of symptomatic disease despite having moderate biochemical disease activity. Of note, biochemical-only relapse/progression, which is marked by the reappearance or increase in the amount of measurable paraprotein without disease symptoms, can signify disease progression and warrant further treatment.12 As there were concerns on the lack of treatment options, the compassionate use of daratumumab was applied in mid-2020. However, the patient has declined switching to a new regimen since he was doing well on ixazomib-based treatment for almost 3 years.
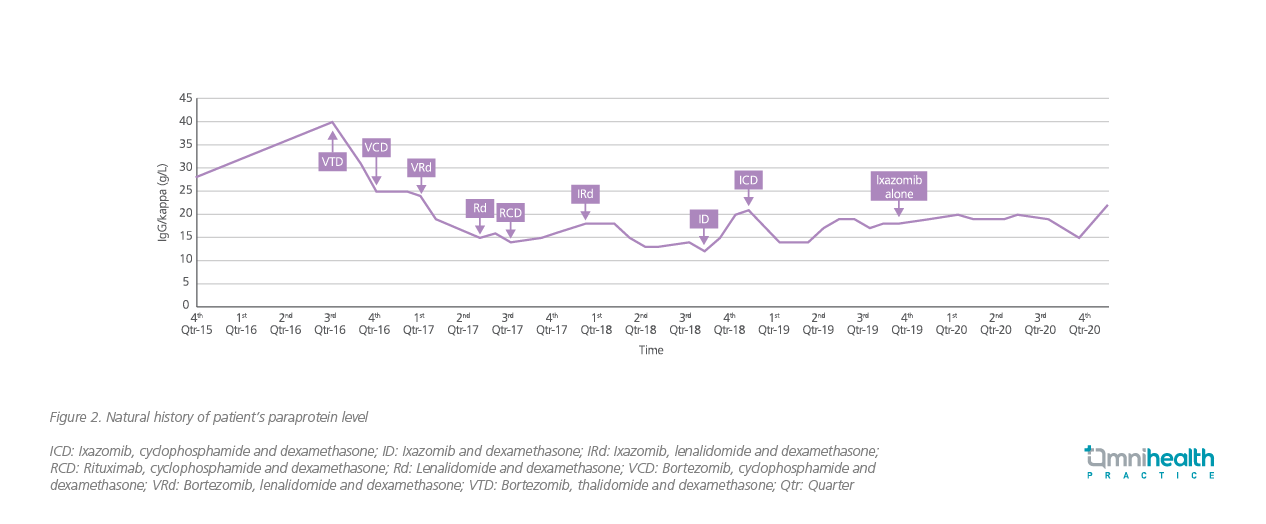
ICD: Ixazomib, cyclophosphamide and dexamethasone; ID: Ixazomib and dexamethasone; IRd: Ixazomib, lenalidomide and dexamethasone; RCD: Rituximab, cyclophosphamide and dexamethasone; Rd: Lenalidomide and dexamethasone; VCD: Bortezomib, cyclophosphamide and dexamethasone; VRd: Bortezomib, lenalidomide and dexamethasone; VTD: Bortezomib, thalidomide and dexamethasone; Qtr: Quarter
Discussion
Based on the demonstrated survival benefit of proteasome inhibitors from the ASPIRE, TOURMALINE-MM1 and other similar studies, the treatment paradigm of rrMM has shifted towards continuous from fixed cycle treatment.13 In particular, prolonged disease control is now often achievable especially with continuous lenalidomide-based combination therapies.13 However, while continuous lenalidomide treatment is preferred if resources allow, the current Samaritan fund policy can only support a finite number of cycles of lenalidomide which has limited its use for many less privileged patients. Fortunately, with the recent inclusion of the IRd combination into the Community Care fund, IRd is expected to become increasingly popular in the early treatment of rrMM.
It is usually very difficult to achieve disease control when a MM patient becomes refractory to lenalidomide following doublet treatment with Rd. With the emergence of effective triplet regimens such as IRd, MM patients can now enjoy an additional treatment option when their disease relapse or become refractory. When prescribing IRd, a continuation of treatment until disease progression or unacceptable toxicity is usually recommended. However, IRd should be prescribed to frail elderly patients with caution due to the potential of introducing additional morbidities. As demonstrated in this local case, a continuous ixazomib-based treatment had achieved disease control for almost 3 years, which was longer than the PFS of 20.6 months as observed in the TOURMALINE-MM1 trial, even when the patient did not achieve PR during the induction therapy and chose not to receive ASCT.3 That said, dexamethasone can be omitted from the IRd regimen after the initial phase of treatment if good response is achieved as steroid-related side effects are not uncommon at the prescribed dose.
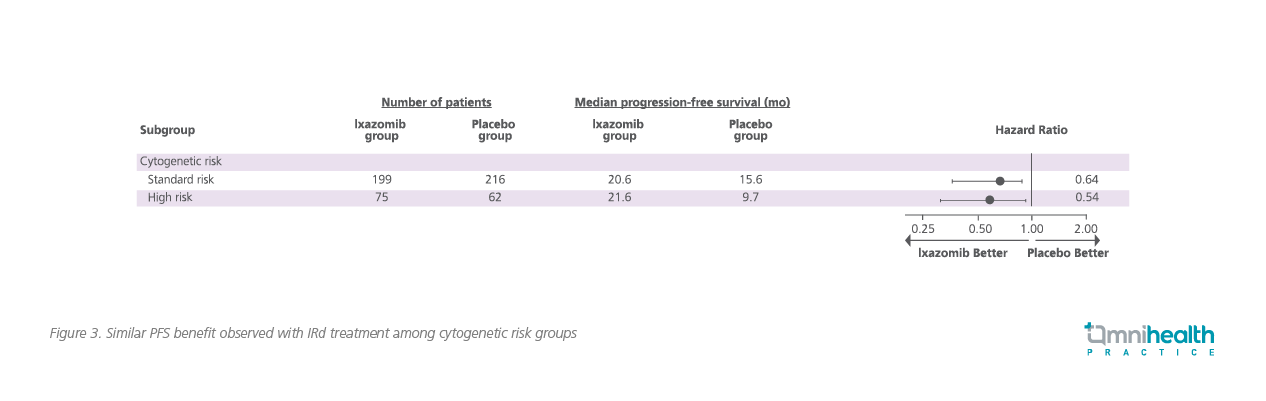
Whenever resources are available, patients with high-risk cytogenetic abnormalities are recommended to receive more potent combinations, including proteasome inhibitors plus immunomodulatory drugs for induction, and ASCT when eligible followed by maintenance therapy. However, while high-risk cytogenetics including abnormalities in chromosome 1q are routinely tested at diagnosis, repeated testing after disease relapse is seldom performed as it is not a standard clinical service in the Hong Kong Hospital Authority setting. In this regard, IRd might be a viable option to close this gap since it has demonstrated equal effectiveness regardless of the underlying cytogenetics, with a similar median PFS benefit of 21.4 months in rrMM patients with high-risk cytogenetic features versus 20.6 months in those with standard-risk cytogenetic features as observed in the TOURMALINE-MM1 trial.3 When compared to patients with high-risk cytogenetic features in the placebo arm, IRd also demonstrated an improved PFS of 11.7 months (21.4 vs. 9.7 months, HR=0.54; 95% CI: 0.32-0.92; p=0.02) (Figure 3).3 Even in those with del(17p) or t(4;14) without del(17p) and t(14;16) aberrations, PFS benefits of 21.4 and 18.5 months, respectively, were observed with IRd.3 Where high-risk cytogenetics may not be tested for relapsed patients in Hong Kong, IRd triplet therapy could be prescribed in the early treatment of rrMM.
In addition to efficacy, the quality-of-life advantages of IRd oral combination therapies were well demonstrated in this shared case. Without the need for drug administration in a hospital setting, ixazomib was also well-tolerated, with only diarrhea and thrombocytopenia being the most commonly encountered adverse events (AE). Similarly in the TOURMALINE-MMA1 trial, IRd had comparable ≥grade 3 AEs with placebo.9 In practice, such AEs are usually easily manageable by dose reduction to 3 or 2.3mg. Although the upper respiratory tract infections, peripheral neuropathy and gastrointestinal AEs including diarrhea, constipation, nausea, and vomiting were more frequently observed in the ixazomib group, they were all relatively easy to manage with symptomatic therapy.9 On the other hand, ixazomib was shown to be able to maintain the physical, emotional and social function domains of quality of life throughout its treatment course.14 Although a deepened treatment response was not observed in this local case with time, Dr. Chau concluded that “It is apparent that continuous treatment with ixazomib was effective in maintaining a stable asymptomatic state for nearly 3 years and enabling the patient to continue with his favorite sport activities.”
Conclusion
Although the integration of ASCT, proteasome inhibitors and immunomodulatory drugs has significantly improved the survival and quality of life for MM patients, most of them would eventually experience relapses or refractory diseases that would require further treatment. Especially in those who have chosen not to undergo ASCT due to concerns of treatment risks, a continuous triplet combination treatment would be required to maintain disease control. In addition to the survival benefit demonstrated in the TOURMALINE-MM1 trial, the continuous use of ixazomib-based treatment in this real-world case has shown PFS efficacy, while maintaining a good quality of life for the patient who did not undergo ASCT. With its effectiveness consistent across cytogenetic risk groups, ixazomib is a well-tolerated treatment option for rrMM that could be widely adopted.

