CASE REVIEW
Local experience sharing: The sustained clinical benefits of ixazomib in long-term rrMM management
Nowadays, continuous treatment has become more commonplace as opposed to the limited cycles of therapy to help relapsed/refractory multiple myeloma (rrMM) patients achieve and remain in remission. However, since patients in the relapsed/refractory (rr) state are generally older and frailer, they are less likely to tolerate more rigorous intravenous (IV) treatments over extended periods of time.1 Oral therapy is the next generation of long-term rrMM management.1 Thus far, ixazomib + lenalidomide + dexamethasone (IRd) is the first-and-only all-oral regimen proven for the treatment of rrMM patients. Its efficacy had been demonstrated in the TOURMALINE-MM1 study and backed by data from the real-world (RW) cohorts. Thanks to the compelling evidence and the edge of convenience, the IRd regimen has been widely adopted in clinical practice and helped bring the disease under control for managing rrMM patients. In an interview with Omnihealth Practice, Dr. Lau, Chi-Kuen shared a clinical case of an rrMM patient achieving a very good clinical response and has been on the IRd treatment for more than 3 years, demonstrating the effectiveness of IRd in local practice.
Background
The rrMM management should not solely focus on avoiding biochemical relapse.1 Rather, preventing multiple myeloma (MM)-related complications, such as anemia, hypercalcemia, bone pain, and infections, are also crucial for rrMM patients.1 According to Dr. Lau, continuous rrMM treatment has become more popular in recent years to help patients remain in clinical and biochemical remission. Nevertheless, MM patients who have relapsed may require more potent treatment options. The oral administration of ixazomib offers an alternative to traditional rrMM management, which is often administered intravenously. Dr. Lau also shared his experience in using IRd combination in rrMM treatment, demonstrating the safety and efficacy of IRd in a local clinical setting.
Case review
A 70-year-old female patient with a history of type 2 diabetes mellitus (T2DM) and ischemic heart disease (IHD) was diagnosed with International Staging System (ISS) stage 3 MM in 2011. In the absence of any extramedullary disease, she was assessed to have an Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 3, indicating the limited ability of self-care and ≥50% waking hours confined to chair or bed. No genetic workup had been performed since the initial diagnosis. Cyclophosphamide and dexamethasone (Cy-dex) were prescribed as the initial treatment, and a partial response (PR) for 1.5 years was achieved.
In 2017, the patient had a symptomatic relapse with the chief complaint of bone pain. Upon investigation, she was also found to have mild anemia with a hemoglobin (Hb) level of 10g/dL. She also had an elevated paraprotein level [Immunoglobulin G-κ (IgG-κ)] of 23g/L and presented with mild hypercalcemia at 2.65mmol/L. This constituted a biochemical relapse of MM, despite the patient’s normal renal function. Subsequently, she was switched from the Cy-dex regimen to the IRd regimen. High efficacy aside, the decision of switching the treatment to ixazomib was also based on the lower cardiovascular (CV) event risk associated with ixazomib since the patient had baseline CV comorbidities. In addition, ixazomib was the only oral therapy for MM management, and its convenience was believed to be particularly beneficial to patients with poor performance status.
Then, the patient’s blood biochemical parameters returned to normal values soon after the initiation of IRd treatment. Her anemia was resolved with the Hb level normalizing to 12g/dL, and the blood calcium levels returning to within the normal range. The IgG-κ levels also dropped to <3g/L after 3 cycles of IRd regimen. In addition, a skeletal survey was performed, with no detection of lytic bone lesion (figure 1 and 2). Her chief complaint of bone pain was completely resolved after a few months of IRd treatment.
The patient has been still on the IRd treatment as of 2021 with a very good response. While remaining in remission, she has not been experiencing any severe adverse events (AEs) relating to the treatment. “Ixazomib is a potent rrMM treatment welcomed by patients who embrace its convenience and improved quality of life (QoL),” Dr. Lau concluded.
Discussion
High efficacy of IRd demonstrated in clinical trial
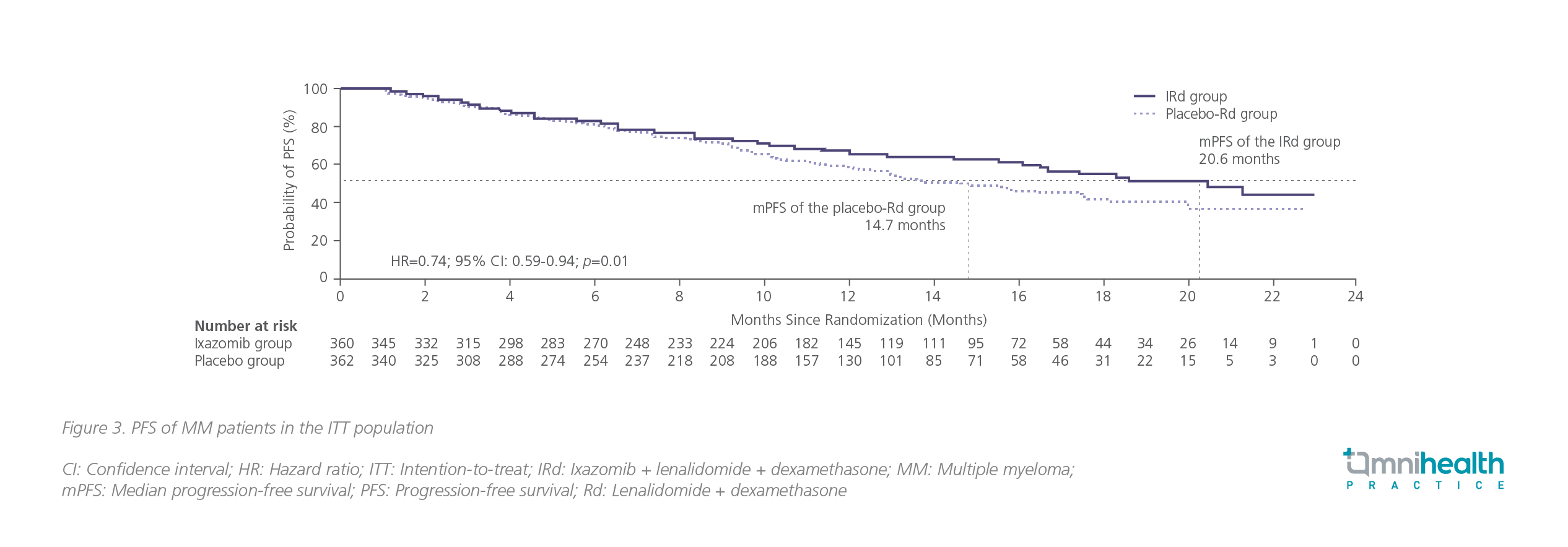
The clinical efficacy of the IRd combination was first demonstrated in the TOURMALINE-MM1 trial, which enrolled rrMM patients with an ECOG-PS of 0-2 and who had received 1-3 prior lines of therapy.2 The participating patients were randomized to receive either ixazomib 4mg (n=360) or placebo (n=362) orally on days 1, 8 and 15.2 Both arms were administered with lenalidomide 25mg and dexamethasone 40mg tablets concomitantly.2
The primary endpoint was median progression-free survival (mPFS), in which the IRd group had a significant improvement of 35% over the placebo-Rd group (20.6 months vs. 14.7 months; HR=0.74; 95% CI: 0.59-0.94; p=0.01) with a median follow-up of 14.8 months in the ixazomib group (figure 3).2
In a recent update with a median follow-up of 85 months, the median overall survival (mOS) with IRd was shown to be numerically longer than that of the placebo group (53.6 months vs. 51.6 months; HR=0.939; 95% CI: 0.784-1.125; p=0.495), but without statistical significance.3 In certain predefined subgroups, which included patients who were aged >65-75 years (HR=0.757) having a poorer baseline status (HR=0.779), or having 2 or 3 prior lines of treatment (HR=0.845), the benefits of risk reduction in death were more profound.3
Remarkable survival benefits of IRd over placebo unveiled in RW cohorts
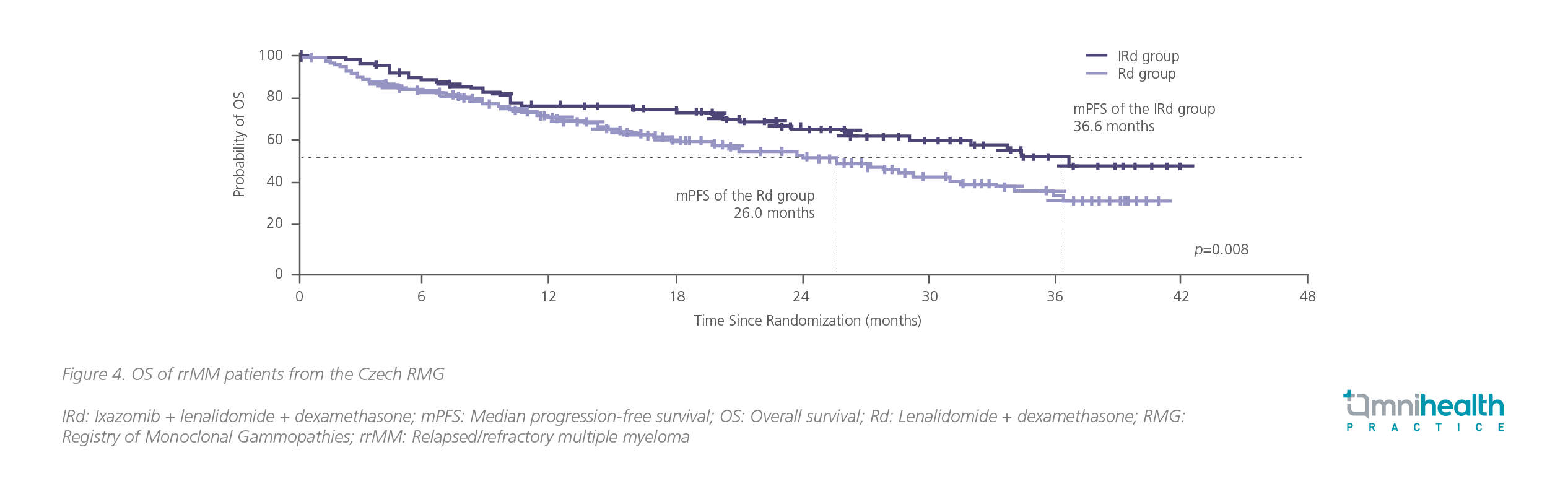
The effectiveness of IRd was revealed in several multicenter, RW studies. The RW data drawn from the Czech Registry of Monoclonal Gammopathies (RMG) with a total of 344 patients being treated with IRd (n=127) or lenalidomide + dexamethasone (Rd) (n=217) showed an mPFS of 23.1 months with IRd vs. 11.6 months in the Rd group (HR=0.67; 95% CI: 0.51-0.89; p=0.006) for patients within relapse 1-3.4 The progression-free survival (PFS) advantage with IRd over Rd was translated into an improved overall survival (OS) of 36.6 months for patients treated with IRd vs. 26.0 months for patients treated with Rd (p=0.008) (figure 4).4
Limitations of clinical trials and importance of RW evidence for rrMM management
There has been growing appreciation of RW data in the field of rrMM management, since clinical trial data do not always translate into RW outcomes.4,5 One of the major reasons was that a large proportion of real-life rrMM patients with certain baseline characteristics were not enrolled in clinical trials, leading to bias in the trial outcomes and a potentially large gap with the RW data. Reports of some RW analyses suggested that approximately 40% of RW patients were excluded from phase 3 trials due to their lower 3-year survival rate of 63% vs. 70% for patients who were eligible (p=0.0392).5,7 In addition, since MM is a disease which is more commonly diagnosed at an advanced age, patients are more likely to have multiple comorbidities and organ function declines, which are regarded as the vital factors under-represented in clinical trials.7 As such, it is of paramount importance for physicians to understand the limitations of the trial outcomes and the differences between the RW and clinical trial populations.
High tolerability and convenience make IRd a suitable, long-term treatment option
In the TOURMALINE-MM1 trial, limited additional toxicity was reported with the IRd regimen compared with placebo.2 The incidence of grade ≥3 AEs with IRd (n=267, 74%) was only slightly higher than that of the placebo group (n=247, 69%).2 The rate of cardiotoxicity was also similar between the 2 arms with the rate of heart failure (n=16, 4%) vs. (n=14, 4%) and myocardial infarction (n=5, 1%) vs. (n=8, 2%) for the ixazomib and placebo groups, respectively.2
Being an all-oral regimen, ixazomib may be able to circumvent frequent hospital stays and infusion-related events that are more common with the traditional rrMM management.4 In addition, RW studies have shown that IRd did not increase the rate of treatment termination due to AEs when compared with Rd (3.1% vs. 4.1%), implying that the IRd treatment, apart from being more convenient, was also more tolerable in the real-life clinical practice.4,8
Dr. Lau reminded that the CV risks in rrMM patients should also be assessed carefully, since most of the existing proteasome inhibitors (PIs) are associated with an increased risk of CV events. In patients with baseline CV risks, ixazomib is preferred due to its relatively lower risk of cardiotoxicities among other PIs.
Since the current practice of rrMM management has been shifted from limited cycles of therapy to extended periods of continuous treatment, it is important to provide a convenient and safe option for patients to maintain their QoL, particularly those who are not ambulatory or cannot tolerate IV administration. “Patients’ preference is also a major consideration when making a treatment decision,” Dr. Lau added.
Conclusion
A long-term maintenance therapy is required for good disease control in rrMM patients. The all-oral ixazomib-based regimen, which has demonstrated sustained efficacy and safety in both clinical trials and RW studies, can be a suitable treatment option for rrMM patients to manage the disease in the long run.
This is an independent editorial article, published and distributed through unrestricted educational support from Takeda Pharmaceuticals (HK) Ltd, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher, and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Lee JH. Et al. Treatment of relapsed and refractory multiple myeloma. Blood Res. 2020;55:S43-S53.
- Moreau P. et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016; 374:1621-34.
- Richardson P.G. et al. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients with Relapsed or Refractory Multiple Myeloma. J Clin Oncol 39:2430-2442.
- Minarik J. et al. Survival benefit of ixazomib, lenalidomide and dexamethasone (IRD) over lenalidomide and dexamethasone (Rd) in relapsed and refractory multiple myeloma patients in routine clinical practice. BMC Cancer 2021; 21:73
- Terpos E. et al. Real-world effectiveness and safety of ixazomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Ann Hematol. 2020; 99(5):1049-1061.
- Cohen Y.C. et al. Ixazomib-based regimens for relapsed/refractory multiple myeloma: are real-world data compatible with clinical trial outcomes? A multi-site Israeli registry study. Ann Hematol. 2020; 99, 1273-1281
- Chari A. et al. Real-world outcomes and factors impacting treatment choice in relapsed and/or refractory multiple myeloma (RRMM): a comparison of VRd, KRd, and IRd. Expert Rev. Hematol. 2020; 13(4): 421-433
- Hájek R, et al. Closing the Efficacy and Effectiveness Gap: Outcomes in Relapsed/Refractory Multiple Myeloma Patients Treated with Ixazomib-Lenalidomide-Dexamethasone in Routine Clinical Practice Remain Comparable to the Outcomes Reported in the Phase 3 TOURMALINE-MM1 Study. Presented at: 61st Annual Meeting of the American Society of Hematology (ASH); December 7-10, 2019; Orlando, FL, the United States.

