CONFERENCE UPDATE: ESMO 2025
Adjuvant abemaciclib + ET reduces risk of death in HR+, HER2-, high-risk early breast cancer: Primary OS results from the monarchE trial
STUDY DESIGN
In the monarchE trial, two years of adjuvant abemaciclib + endocrine therapy (ET) significantly improved invasive disease-free survival (IDFS) vs. ET alone in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 negative (HER2-), node-positive, high-risk early breast cancer (EBC), establishing this regimen as standard of care.1 At a median follow-up of 54 months, benefits were sustained with persistent separation of IDFS and distant relapse-free survival (DRFS) curves beyond the treatment period, suggesting a carryover effect.1 A favorable overall survival (OS) trend also emerged, where nearly twice as many patients in the ET arm living with metastatic disease compared to those receiving abemaciclib + ET, which suggested a potential OS effect with longer follow-up.1 At a median follow-up of 6.3 years, over 650 deaths occurred in the intention-to-treat (ITT) population, which triggered the primary OS analysis.1
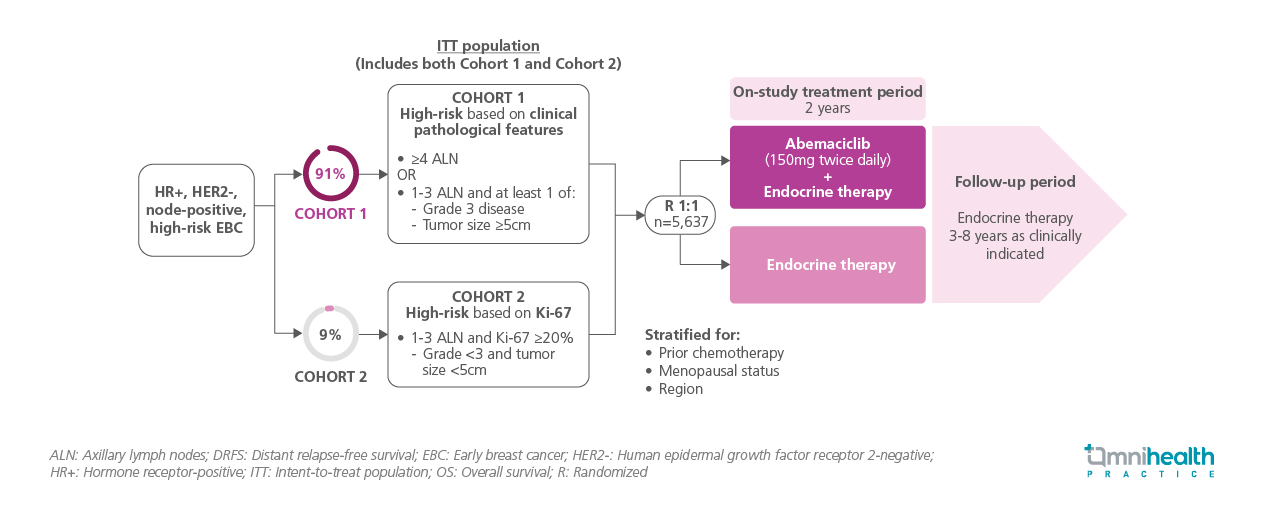
MonarchE was a randomized, open-label, phase 3 trial that enrolled 5,637 patients with HR+, HER2-, node-positive, high-risk EBC across 603 sites in 38 countries between July 2017 and August 2019.1 The ITT population consisted of cohorts 1 (n=5,120) and 2 (n=517).1 Cohort 1 included patients at high risk based on clinical-pathological features: ≥4 positive axillary lymph nodes (ALN) or 1-3 ALN with either grade 3 histology or tumor size ≥5cm.1 Cohort 2 included patients at high risk based on Ki-67 status, defined as 1-3 ALN with Ki-67 ≥20%, grade <3, and tumor size <5cm.1
Patients were randomized 1:1 to receive abemaciclib 150mg twice daily + ET (aromatase inhibitor, tamoxifen, or gonadotropin-releasing hormone [GnRH] agonist) for two years or ET alone.1 ET was continued for 3-8 years as clinically indicated during the follow-up period.1 As of the primary OS data cutoff, all patients had been off abemaciclib treatment for at least 4 years and the median follow-up duration was 76 months.1 The primary OS, and updated IDFS and DRFS in the ITT population were presented.1
| Primary endpoint: |
|
| Key secondary endpoints: |
|
| Safety: |
|
“Abemaciclib is the first CDK4/6 inhibitor to achieve a statistically significant improvement in OS for patients with HR+ HER2-, node-positive, high-risk EBC.”
Professor Stephen Johnston
The Royal Marsden,
NHS Foundation Trust,
London, United Kingdom

