CONFERENCE UPDATE: ESGO 2024
Long-term HRQoL outcomes of dostarlimab + chemotherapy in patients with primary advanced or recurrent EC from the RUBY trial
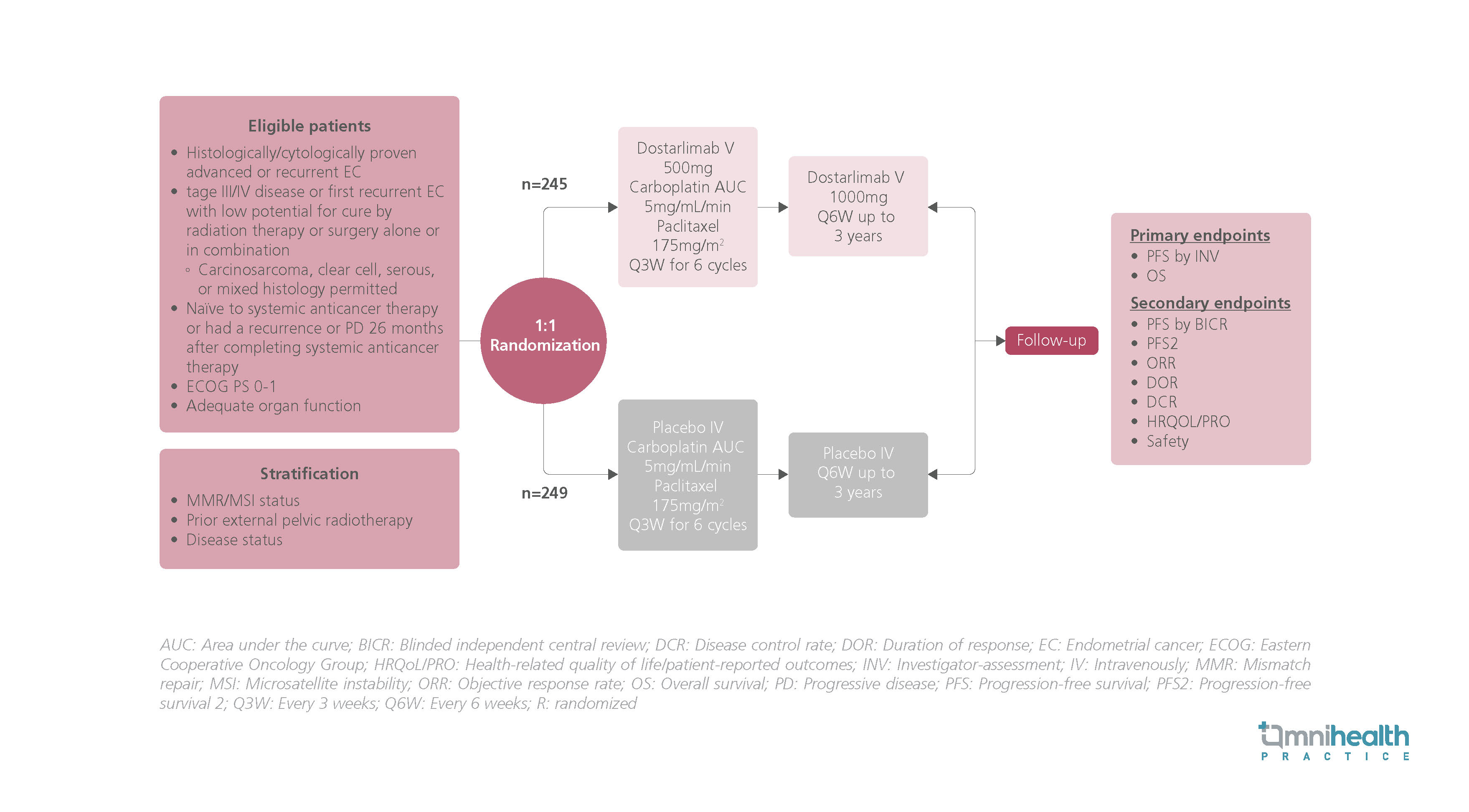
STUDY DESIGN
Dostarlimab in conjunction with carboplatin-paclitaxel (CP) has previously been shown to significantly improve progression-free survival (PFS) and overall survival (OS) outcomes compared with CP alone in patients with primary advanced or recurrent endometrial cancer (EC).1 This has led to its approval in the first line for EC patients in the mismatch repair deficient (dMMR) microsatellite instability-high (MSI-H) population.1 However, more data are required to describe the health-related quality of life (HRQoL) of this immunotherapy CP combination over extended periods.1
Previously, the RUBY trial included 494 patients who had stage III/IV EC or first recurrent EC.1 They were randomized 1:1 to receive dostarlimab 500mg (n=245) or a matching placebo (n=249) in addition to 6 cycles of carboplatin at area under the curve (AUC) of 5mg/mL/min and paclitaxel 175mg/m2 Q3W.1 This was followed by dostarlimab 1,000mg Q6W or a matching placebo for up to 3 years.1 The primary endpoints included PFS by investigator assessment and OS outcomes.1 Secondary endpoints included PFS by blinded independent central review (BICR), PFS2, objective response rate (ORR), duration of response (DOR), disease control rate (DCR), HRQoL outcomes, as well as safety results.1
In this post-hoc analysis, the results of time-to-deterioration (TTD) of patient-reported outcomes (PROs) on HRQoL over time were been reported, allowing longitudinal comparisons of the trial arms.1 The analysis was conducted using the European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life questionnaire (QLQ-C30) and the Endometrial Cancer Module (QLQ-EN24).1 Time to first deterioration (TTD1) was defined by the first ≥10 points deterioration from baseline while time to permanent deterioration (TTDP) was defined by a deterioration of ≥10 points sustained at all subsequent time points.1
FINDINGS
| TTD1 outcomes: |
|
|
|
|
|
| TTDP outcomes: |
|
|
|
|
|
“These findings, together with the PFS benefits, further support the use of dostarlimab + CP as a standard of care in patients with primary advanced or recurrent EC.
Dr. Ingrid Boere
Department of Medical Oncology,
Erasmus MC Cancer Centre,
The Netherlands

