CONFERENCE UPDATE: ESGO 2024
Final analysis of NORA demonstrates the OS benefits of niraparib maintenance in Chinese PSROC patients regardless of gBRCAm status
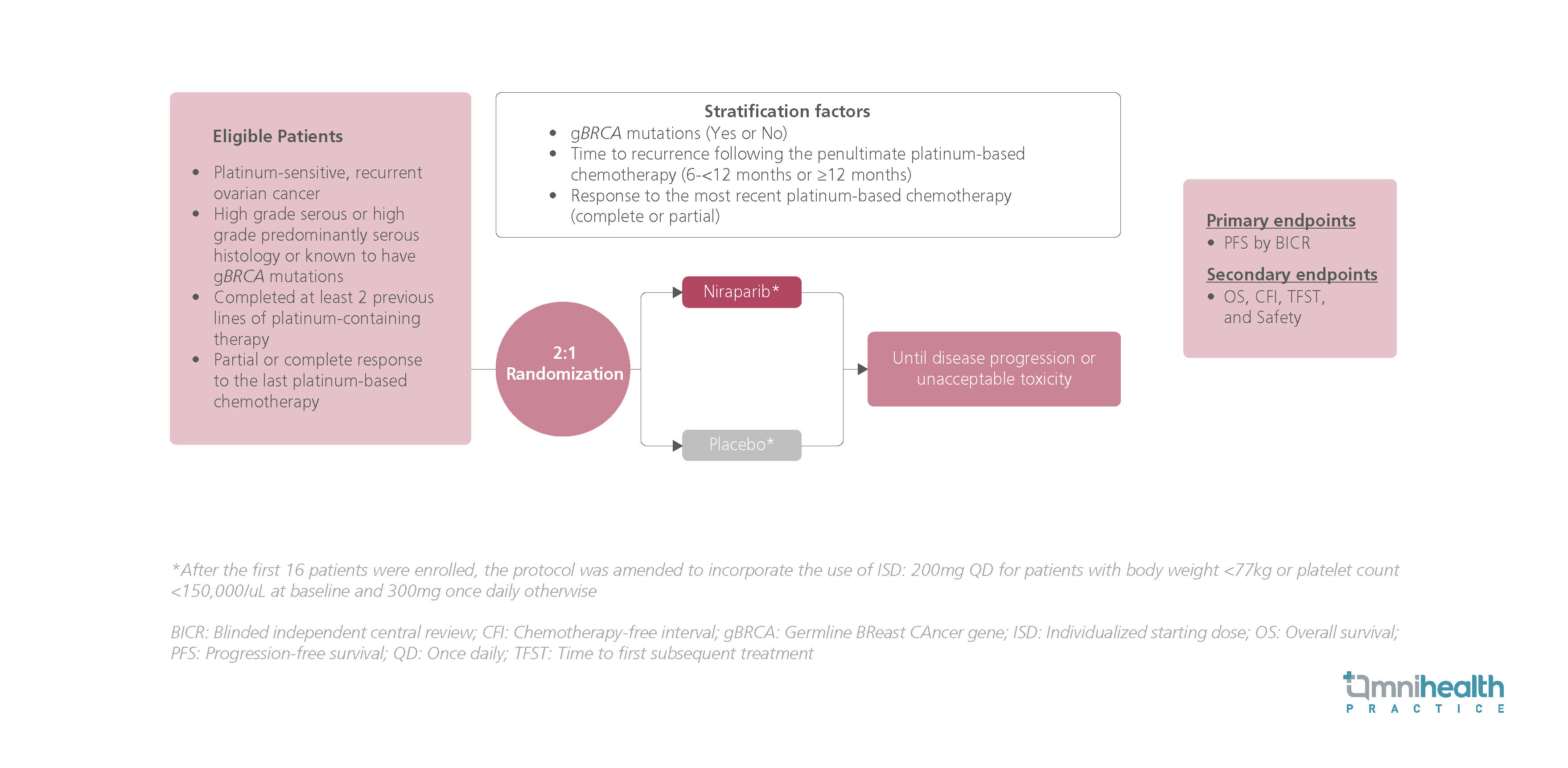
STUDY DESIGN
Niraparib is a potent and highly selective poly-ADP ribose polymerase (PARP) inhibitor.1 PARP inhibitors have shown a trend of favorable overall survival (OS) in platinum-sensitive recurrent ovarian cancer (PSROC) in the maintenance setting.1 However, this OS benefit appears inconsistent in patients without BReast CAncer gene (BRCA) mutations which has been attributed to confounding factors, such as post-progression therapies.1
The Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA) trial is a randomized, double-blinded, placebo-controlled phase 3 study conducted in the Chinese population.1 The trial assessed niraparib as a maintenance therapy in PSROC using an individualized starting dose (ISD).1 Participants had high-grade serous, high-grade predominantly serous histology or germline BRCA mutations (gBRCAm) and had completed ≥2 previous lines of platinum-containing therapy with a partial or complete response.1 They were randomized 2:1 to receive niraparib or a matching placebo and were dosed according to an ISD.1 Patients received a 300mg QD dose unless they had a body weight of <77kg and/or a platelet count of <150,000/μL, in which case 200mg QD was used instead.1
The primary endpoint of the study was progression-free survival (PFS) by blinded independent central review (BICR).1 Secondary outcomes included OS results as well as the chemotherapy-free interval (CFI), the time to first subsequent treatment (TFST), as well as safety outcomes.1 At the primary analysis, niraparib has shown significant improvement over placebo in terms of PFS regardless of gBRCAm status.1 Another interim analysis revealed OS benefits across gBRCAm status with the use of ISD.1 In the captioned analysis, the results of the final OS analysis from NORA have been presented.1 Additionally, the safety analysis in terms of incidences of myelodysplastic syndrome (MDS), acute myeloid leukemia (AML) and other second primary malignancies were also summarized.1
| OS: |
|
|
|
|
|
| Safety: |
|
|
|
“The phase 3 NORA trial continues to support the use of niraparib maintenance therapy with ISD for patients with PSROC, regardless of gBRCAm status.”
Dr. Wu Xiaohua
Fudan University Shanghai Cancer Center

