CONFERENCE UPDATE: ESMO 2023
Adjuvant abemaciclib + ET reduces the risk of invasive disease and relapse at 5-years in HR+, HER2- high-risk EBC: Interim analysis of the monarchE trial
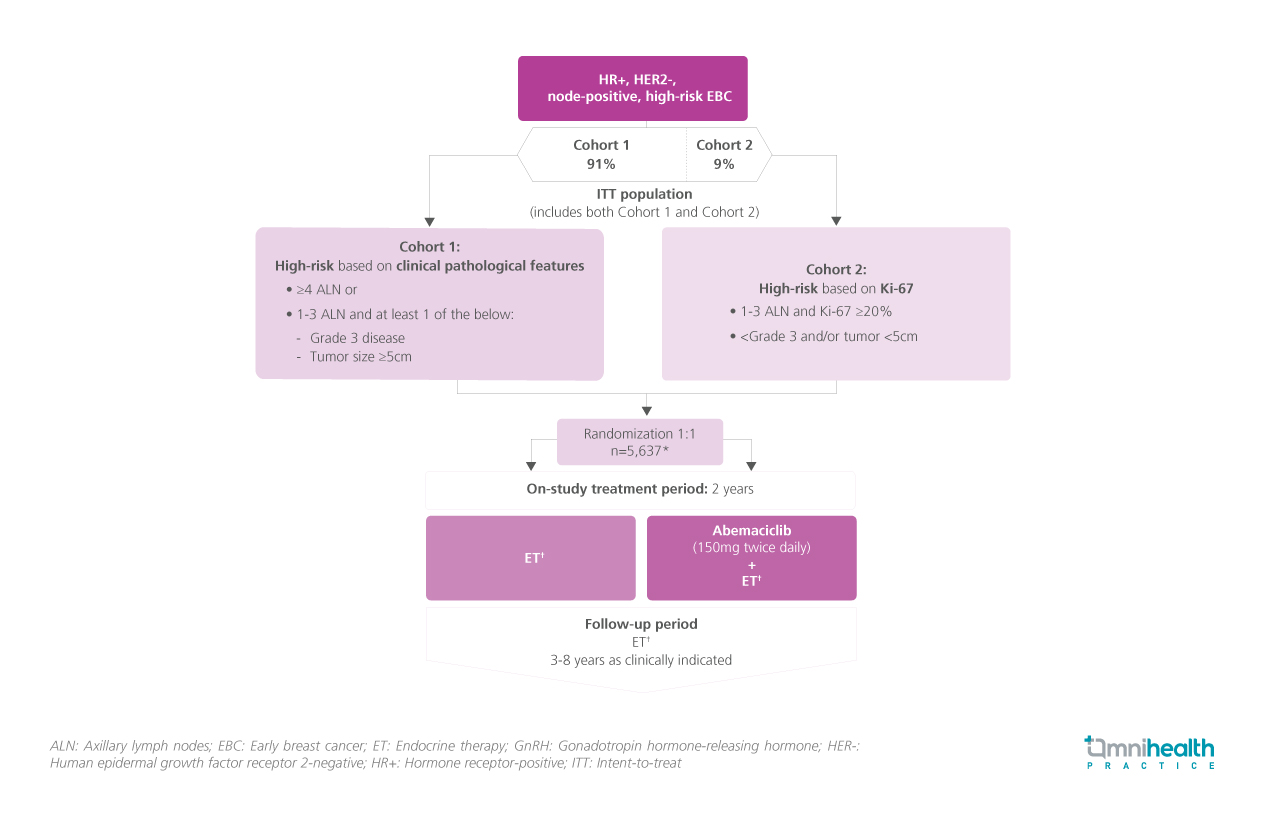
STUDY DESIGN
Abemaciclib is a CDK4/6 inhibitor and a globally approved, standard adjuvant therapy for patients with node-positive, early breast cancer (EBC) who are at high risk of recurrence.1 In the monarchE trial, the clinical efficacy of adjuvant abemaciclib + endocrine therapy (ET) was evaluated among patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) high-risk EBC.1
5,637 eligible patients with HR+, HER2-, node-positive, high-risk EBC who were classified as high-risk based on either clinical pathological features (≥4 axillary lymph nodes or grade 3 disease or tumor size ≥5cm) (n=5,120) or the Ki-67 index (<4 axillary lymph nodes and Ki-67 ≥20%) (n=517) were recruited and randomized to receive abemaciclib (150mg twice daily) + ET or ET alone for 2 years.1 After the 2-year on-study treatment period, both arms received ET for an additional 3-8 years.1 At the latest data cutoff date (3 July 2023), the median follow-up was 54 months with more than 80% of patients being followed up for at least 2 years since completing the course of abemaciclib.1
At the pivotal 5-year milestone of the monarchE trial, the primary endpoint of the prespecified interim analysis was invasive disease-free survival (IDFS), whilst the secondary endpoints presented were IDFS in high Ki-67 populations, overall survival (OS), and distant relapse-free survival (DRFS).1 Safety outcomes were also measured.1
FINDINGS
| Primary endpoint: |
|
|
|
| Secondary endpoints: |
|
|
|
|
| Safety: |
|
|
"These data are consistent with a carryover effect and further support the addition of adjuvant abemaciclib to ET for patients with HR+, HER2-, node-positive, high-risk EBC"
Dr. Nadia Harbeck
Breast Center,
Ludwig Maximilians University Hospital,
Munich, Germany

