CONFERENCE UPDATE: ESMO 2025
Alectinib prolongs OS and shows durable response in previously untreated advanced ALK+ NSCLC: Final OS analysis from the phase 3 ALEX study
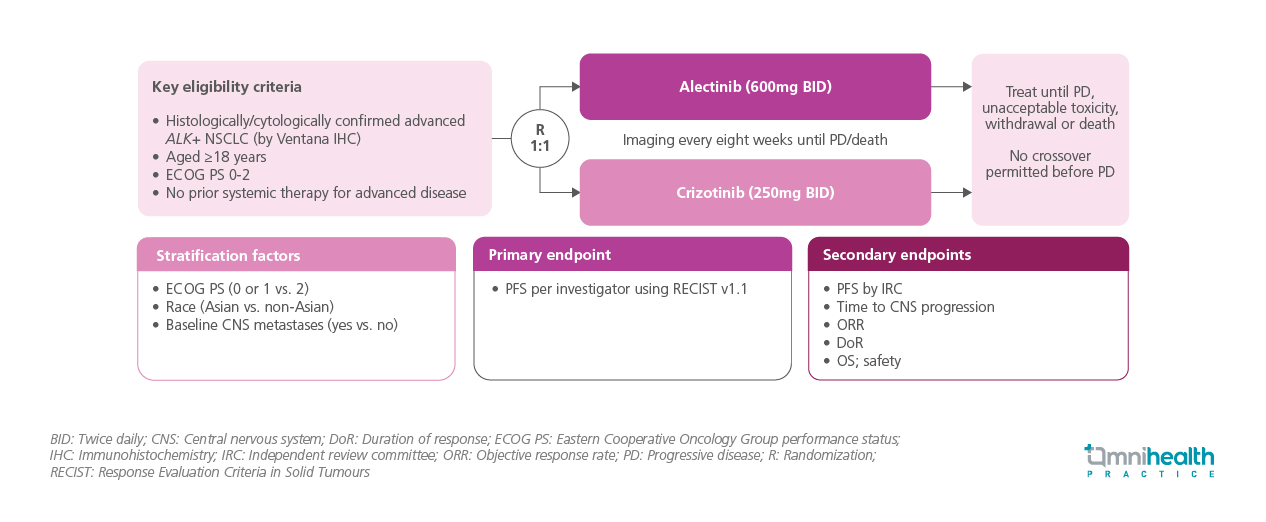
STUDY DESIGN
The ALEX study established alectinib as the first-line treatment for advanced anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC).1 Earlier analyses demonstrated progression-free survival (PFS) advantage with alectinib, with investigator-assessed median PFS of 34.8 months vs. 10.9 months with crizotinib (p<0.0001).1 Despite this clear benefit, overall survival (OS) results remained immature, as median OS was not reached for alectinib after a median follow-up of 48.2 months, while it was 57.4 months with crizotinib (stratified hazard ratio [HR]=0.67; 95% CI: 0.46-0.98).1 Building on these findings, the present analysis provides the final OS and updated long-term safety outcomes following an additional six years of follow-up.1
ALEX was a randomized, open-label, phase 3 trial evaluating first-line alectinib vs. crizotinib in patients with untreated advanced ALK+ NSCLC.1 A total of 303 patients aged ≥18 years with Eastern Cooperative Oncology Group performance status (ECOG PS) 0-2 disease who had not received prior systemic therapy for advanced disease were enrolled.1 Baseline central nervous system (CNS) metastases and prior brain radiation were allowed.1 Patients were stratified by ECOG PS, race, and CNS metastases status, and were subsequently randomized 1:1 to receive alectinib 600mg twice daily or crizotinib 250mg twice daily until progression, unacceptable toxicity, withdrawal, or death.1 Imaging was performed every eight weeks, and crossover prior to confirmed progression was not permitted.1 Baseline characteristics were generally balanced between treatment arms.1
The presentation reported the final OS, duration of response (DoR), as well as updated long-term safety outcomes, after an additional 6 years of follow-up from the previous OS analysis.1
FINDINGS
| Key efficacy endpoints: |
|
| Safety : |
|
“The clinically meaningful OS and prolonged median DoR continue to support first-line alectinib as a standard of care for patients with advanced ALK+ NSCLC.”
Professor Mok, Shu-Kam Tony
Associate Dean (Translation and Entrepreneurship),
Chairman, Department of Clinical Oncolog,
Li Shu Fan Professor of Clinical Oncology,
The Chinese University of Hong Kong
Fellow of ASCO (FASCO)

