Undiagnosed chronic kidney disease (CKD) is a growing concern, particularly among individuals with baseline cardiovascular (CV) comorbidities.1 To effectively maintain kidney health, annual screenings should not focus solely on the estimated glomerular filtration rate (eGFR) but should also incorporate the urine albumin-creatinine ratio (uACR).2 In a recent webinar, Professor Christoph Wanner from the University Hospital of Würzburg, Germany, emphasized the critical need for early diagnosis and treatment of CKD to enhance patient outcomes and quality of life (QoL). He discussed the role of empagliflozin, a sodium-glucose cotransporter 2 inhibitor (SGLT2i), in managing CKD, highlighting its broad implications for various cardio-renal-metabolic (CRM) conditions and helping reduce associated risks.3 Based on the EMPA-KIDNEY trial, he noted empagliflozin’s efficacy not only in patients without albuminuria but also across a diverse range of nephropathies, including frail and elderly individuals.4 He concluded by urging the adoption of current guidelines in clinical practice to combat the increasing burden of CKD worldwide.2
Undiagnosed CKD: A growing concern
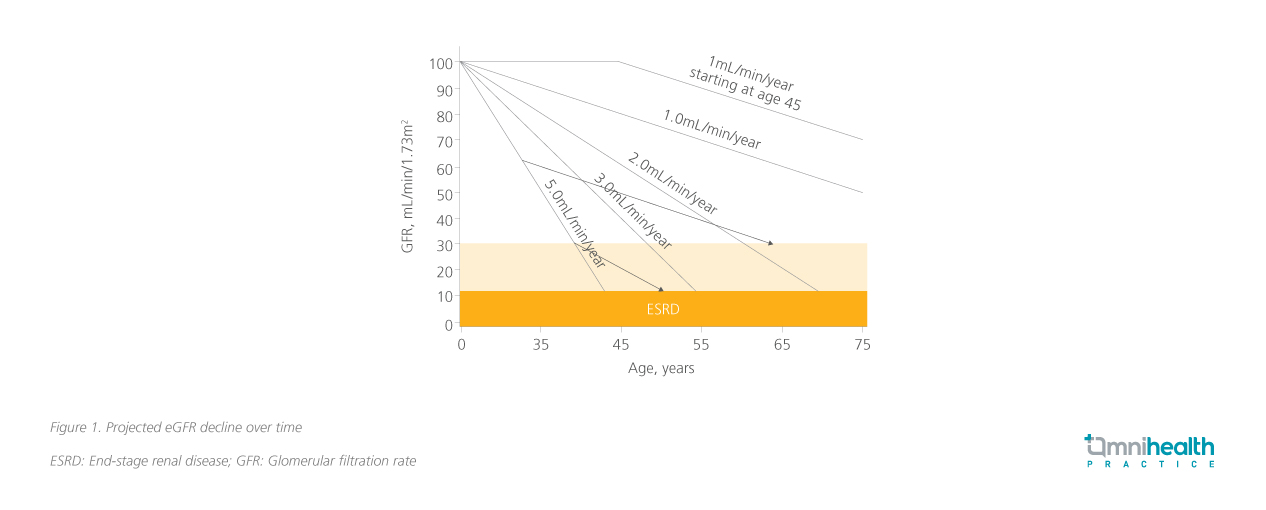
The prevalence of undiagnosed CKD is alarmingly high, particularly among patients with CRM conditions such as CV disease (CVD), hypertension, and diabetes.1 Reports indicate that undiagnosed CKD may be as high as 95% in these high-risk populations.1 Despite the risk of adverse renal outcomes in these patients, current diagnostic practices are inadequate and primarily focus only on eGFR.5 However, eGFR alone is insufficient for timely CKD diagnosis, Prof. Wanner emphasized that many patients may not exhibit clear signs of kidney disease until significant damage has occurred, leading to delays in diagnosis and treatment. When medical intervention is initiated early in the disease course, the need for dialysis may be delayed.6 Initiating treatment when renal function is preserved at 60mL/min/1.73m² provides more years before dialysis is needed compared to starting treatment at 30mL/min/1.73m², as illustrated in figure 1.7
The critical role of uACR alongside eGFR in CKD diagnosis
The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend regular screening for at-risk patients.2 Annual screening for CKD, particularly in high-risk groups such as those with diabetes mellitus, should include both eGFR and uACR tests to accurately diagnose CKD.2 However, these guidelines are often inadequately implemented.2 For instance, a retrospective observational study, InspeCKD, found that general practitioners conducted eGFR tests in only about half of their diabetic patients, while dipstick tests for albuminuria were performed in just 21.2%.5 Standardized tests like uACR were rarely conducted in this population.5 Prof. Wanner highlighted that this lack of comprehensive testing hampers effective CKD diagnosis.
One key issue is that eGFR may not show declines until significant kidney damage has occurred, making it less sensitive for early diagnosis.8 In contrast, uACR can detect kidney damage at earlier stages, even when eGFR is still within normal limits, and high levels of albuminuria can often indicate early kidney injury.8,9 Prof. Wanner explained that uACR provides information about the risk of progression to end-stage renal disease (ESRD) and CV events, and while eGFR is indicative of renal function, it does not specifically assess kidney damage, limiting its utility in risk assessment. Therefore, Prof. Wanner asserted that using both uACR and eGFR provides a more comprehensive view of kidney health by integrating assessments of both function and damage.
Empagliflozin: Key to slowing eGFR decline in diverse populations
Recognizing the value of early detection, the next step in CKD management should be early intervention with an appropriate agent. The EMPA-KIDNEY trial included 6,609 CKD patients with a wide range of eGFR and uACR levels, as well as various causes of nephropathy.4,10 It enrolled patients with eGFR ≥45mL/min/1.73m2 to <90mL/min/1.73m2 and uACR ≥200mg/g, or eGFR ≥20mL/min/1.73m2 to <45mL/min/1.73m2 regardless of uACR.4 The study population had a wide range of eGFR, levels of albuminuria and causes of CKD, and were generally representative of the broader population of CKD patients at risk for disease progression.4 In both treatment arms, approximately 34% had eGFR <30mL/min/1.73m2, 48% uACR ≤300mg/g, and 20% were normoalbuminuric (uACR<30mg/g).4 In addition, roughly 54% did not have a history of diabetes, only 27% had prior CVD.4
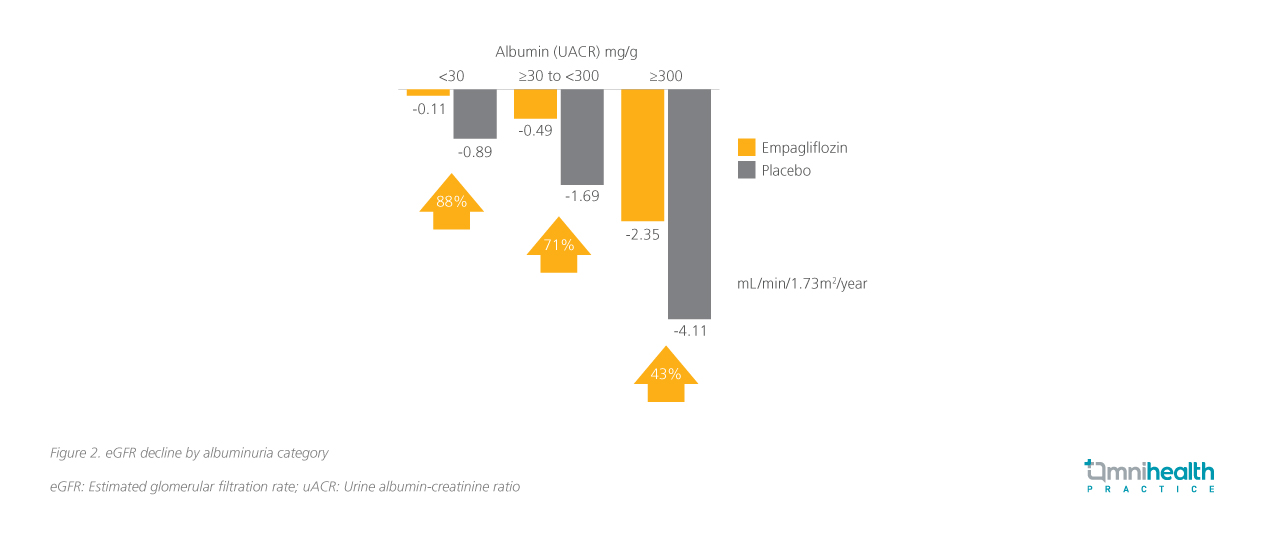
Patients who received empagliflozin experienced a two-fold slower decline in the annual rate of eGFR change compared with those receiving standard-of-care (SoC) alone (-1.37mL/min/1.73m2 vs. -2.75mL/min/1.73m2).4 The effect was even more pronounced in patients with a uACR of <30mg/g, where an 88% difference compared with placebo was observed (figure 2), highlighting the additional benefits of timely treatment with empagliflozin in normoalbuminuric CKD patients.11
Extending the impact of empagliflozin on frail populations
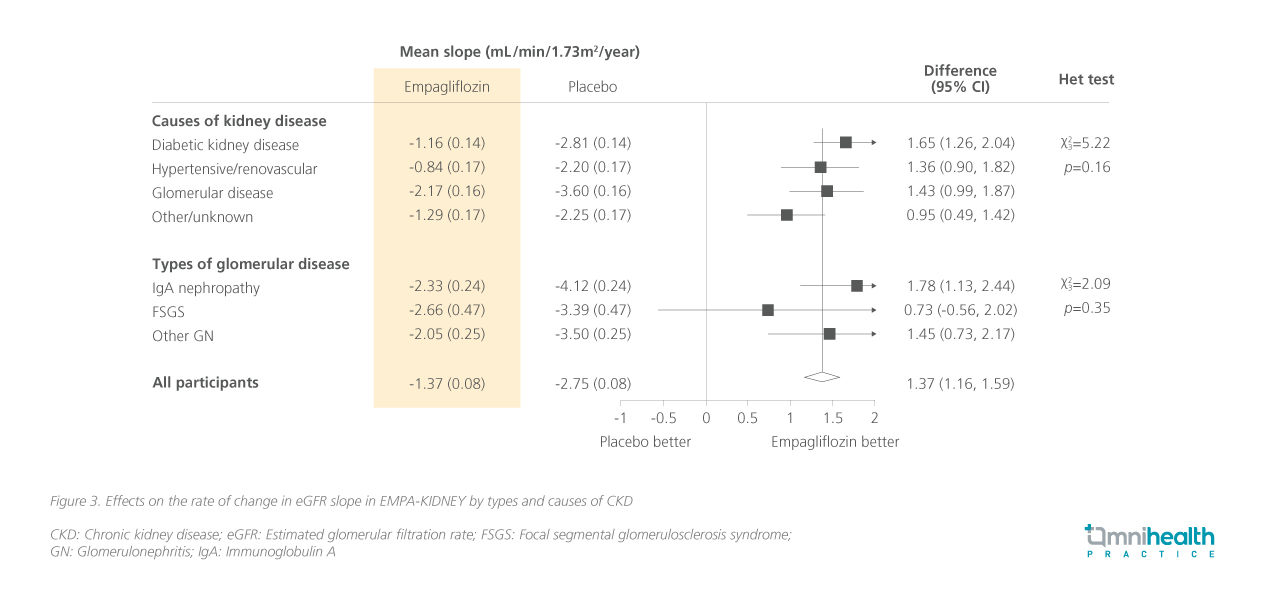
In addition to the benefits for the overall population, the benefits were consistent across select subgroups of patients in the trial, regardless of the cause and type of CKD.12 Figure 3 represents the mean change in the rate of renal function decline across CKD types and causes.12
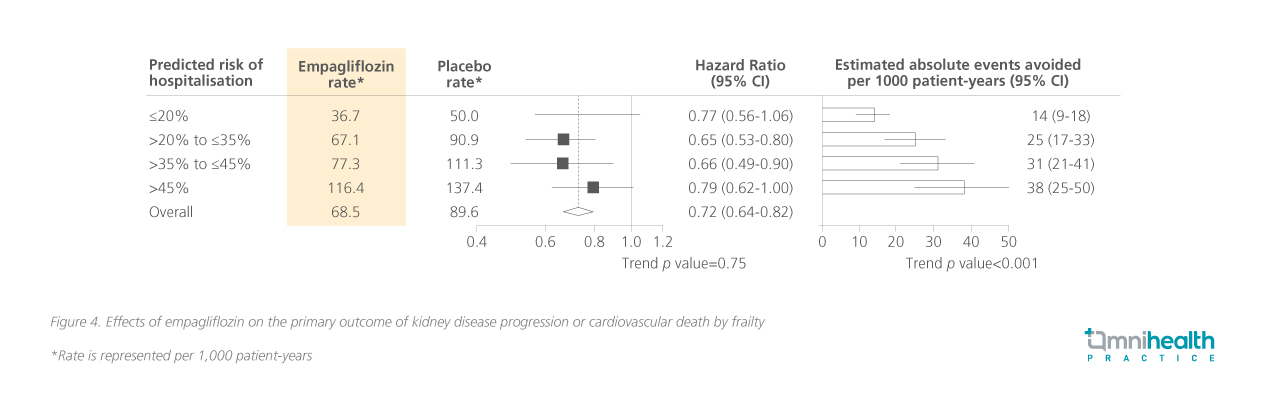
An exploratory analysis of the EMPA-KIDNEY trial focused on frail patients, revealing that renal and CV outcomes were consistent with those in the primary study population maintained.13 The study population was categorized by frailty levels as stratified by the predicted risk of hospitalization (≤20%, >20% to ≤35%, >35% to <45%, and >45%).13 Notable benefits in the primary outcome of kidney disease progression or CV vascular death were observed across different subgroups but were particularly high for those with higher predicted hospitalization risks (figure 4).13 The study also stratified patients by other measures such as polypharmacy, multimorbidity, and health-related quality of life, all of which demonstrated substantial benefits.13 Prof. Wanner highlighted “The frailty status in respect to the primary outcome, hospitalization, the weight against ketoacidosis, fracture and symptomatic dehydration is on the benefit side.” Significant benefits are observed across varying levels of frailty, underscoring the rationale for timely initiation of empagliflozin without delay.13
Evidence-based recommendations from the latest KDIGO guidelines
Commenting on the latest iteration of the KDIGO practice guidelines for non-diabetic CKD in 2022, Prof. Wanner advocated for the use of SGLT2i as a standard of care for managing CKD, particularly in patients with T2D. Based on the results from the EMPA-KIDNEY studies, the following recommendations were made in the KDIGO 2024 guidelines:2
- Recommendation 3.7.1: We recommend treating patients with T2D, CKD, and an eGFR ≥20mL/min/1.73m2 with an SGLT2i
- Recommendation 3.7.2: We recommend treating adults with CKD with an SGLT2i for the following:
- eGFR ≥20mL/min/1.73m2 with uACR ≥200mg/g (220mg/mmol), or
- Heart failure, irrespective of the level of albuminuria
- Recommendation 3.7.3: We suggest treating adults with eGFR 20 to 45mL/min/1.73m2 with uACR <200mg/g (<20mg/mmol) with an SGLT2i
Conclusion
The integration of empagliflozin into CKD management represents a critical advancement in bridging therapeutic gaps across the CKD spectrum, addressing a broad range of patient needs, including those with frailty.3,4,10,13 Findings from the EMPA-KIDNEY trial underscore empagliflozin's unique benefits in the broadest range of CKD patients studied to date, with proven efficacy in slowing eGFR decline in patients with and without albuminuria.4,11 Notably, the impact was especially pronounced in normoalbuminuric CKD patients, emphasizing empagliflozin’s vital role in this subgroup.11 Recent guidelines support the inclusion of empagliflozin, particularly in normoalbuminuric CKD, enabling a more tailored, evidence-based approach to care.2 By prioritizing early detection and dual assessments using uACR and eGFR, healthcare providers can optimize treatment strategies to improve outcomes and QoL for diverse CKD patient populations.
This is an independent editorial article, published and distributed through unrestricted educational support from the pharmaceutical community, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher, and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Tangri N, et al. Prevalence of undiagnosed stage 3 chronic kidney disease in France, Germany, Italy, Japan and the USA: results from the multinational observational REVEAL-CKD study. BMJ Open. 2023;13(5):e067386.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314.
- Zinman B, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117-28.
- Herrington WG, et al. The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127.
- Wanner C, et al. InspeCKD - Analysis of the use of diagnostics in patients at high risk for chronic kidney disease in German general practitioner (GP) practices. MMW Fortschr Med. 2024;166(Suppl 4):9-17. German.
- Fernández-Fernandez B, Sarafidis P, Soler MJ, Ortiz A. EMPA-KIDNEY: expanding the range of kidney protection by SGLT2 inhibitors. Clin Kidney J. 2023;16(8):1187-1198.
- Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol. 2003;14(12):3217-32.
- Matsushita K, et al. CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514-25.
- Cherney DZI, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610-621.
- EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022;37(7):1317-1329.
- Herrington WG, et al. The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127 (supple 1).
- Haynes R. SGLT-2 inhibitors and protection across different CKD aetiologies and stages. Presented at the 25th Hellenic Congress of Nephrology 2024. June 19-21, Athens, Greece.
- Mayne KJ, et al; EMPA-KIDNEY Collaborative Group. Frailty, multimorbidity and polypharmacy: exploratory analyses of the effects of empagliflozin from the EMPA-KIDNEY trial. Clin J Am Soc Nephrol. 2024;19(9):1119–29.


