MEETING HIGHLIGHT
New insights into dapagliflozin: The revolutionary road in diabetic kidney complications
In 2017, approximately 476 million people worldwide were living with diabetes mellitus.1 A study of the International Society of Nephrology revealed that 58% of type 2 diabetes mellitus (T2DM) patients had concomitant chronic kidney disease (CKD), which is also known as diabetic kidney disease (DKD).2 DKD is associated with increased morbidity and mortality risk and has become a huge global burden.3 However, its awareness and diagnosis remain low, and the conventional therapies, such as angiotensin-converting-enzyme inhibitors (ACEIs), lack efficacy data for advanced CKD and are associated with increased risks of hyperkalemia and acute decline in renal function.4,5 Therefore, a novel therapy such as dapagliflozin, an sodium-glucose cotransporter 2 (SGLT2) inhibitor with a prominent reno-protective effect beyond glucose control, is urgently needed. In a recent symposium held virtually in Hong Kong, Professor Mark Cooper shared his insights into the future management of DKD and the exciting results of the DAPA-CKD trial evaluating the long-term efficacy and safety of dapagliflozin in CKD patients with or without T2DM.
Low awareness of DKD and inadequate CKD screening in T2DM patients
“The presence of CKD represents a bad outlook for T2DM patients because it leads to increased mortality and morbidity, so we need to identify these patients early,” Prof. Cooper stated. However, many of these patients remained undiagnosed in real-world clinical practice.6 A large observational study in the United States revealed that although 54.1% of T2DM patients had stage 1-5 CKD, only 12.1% of them were diagnosed by their clinicians, with the diagnoses of stage 1 and 2 CKD being more likely to be missed compared to stages 3-5 CKD.6 In addition, despite the importance of urine protein assessment and the association of high albuminuria with early structural kidney damage, most clinicians still monitored their T2DM patients’ renal function solely based on estimated glomerular filtration rate (eGFR).6 Specifically, more than half of the patients were not routinely tested for urine albumin-creatine ratio (UACR).6 Therefore, with the possibly hidden albuminuria, the prognosis of CKD in the T2DM patients could still be poor even if their eGFR is normal.7 In light of this, most endocrinology societies recommend routine CKD screening in T2DM patients through the assessment of both eGFR and UACR in order to achieve an earlier and more accurate identification of the problem.7
Robust cardiorenal benefits observed for dapagliflozin in the DECLARE-TIMI 58 trial
Preservation of renal function in T2DM patients with CKD by the currently available reno-protective agents is crucial for improving clinical outcomes. Until recently, ACEIs and angiotensin II receptor blockers (ARBs) were the only classes of drugs with proven efficacy in slowing down the progression of CKD.8 However, their increased risks of hyperkalemia or acute renal decline concerned many clinicians, and the mortality rate of DKD patients remained high despite their availability.3,5 A novel therapy with significant reno-protective effect to address the cardiorenal problems is, therefore, urgently needed. “Now, we have a new and exciting treatment for our patients,” Prof. Cooper added. In the DECLARE-TIMI 58 study in which participants had a preserved baseline renal function (Average eGFR=85mL/min/1.73m2, only 30% of patients were albuminuric) and 80% of them were on ACEI or ARB treatment, dapagliflozin, the SGLT2 inhibitor, demonstrated a significant reno-protective effect beyond glucose control by inducing a 47% reduction in the relative risk of a composite renal outcome [Exploratory endpoint, defined as sustained ≥40% decrease in eGFR to <60mL/min/1.73m2, end-stage renal disease (ESRD), and renal death] compared to placebo (n=17,160; HR=0.53; 95% CI: 0.43-0.66, p<0.0001).9 Different from the mechanism of ACEI or ARB which inhibits renin-angiotensin-aldosterone system (RAAS) to cause vasodilation of efferent glomerular arteriole, dapagliflozin contracts the afferent arteriole to reduce hyperfiltration and reduce glomerular hypertension, thus protecting the patients from further kidney injury.10
DAPA-CKD: Long-term renal-specific benefits of dapagliflozin demonstrated in CKD patients
To further evaluate the renal-specific benefits of dapagliflozin in patients with CKD, Dapagliflozin And Prevention of Adverse outcomes in CKD (DAPA-CKD), the only published dedicated renal outcome trial of SGLT2 inhibitors regardless of the T2DM status, was conducted.11 This trial enrolled 4,304 CKD patients with and without T2DM, with a UACR of 200-5,000mg/g, and an eGFR of 25-75mL/min/1.73m2 at screening, on top of a maximum tolerated dose of ACEI or ARB for at least 4 weeks.8
Among all participants, 67.5% (2,906 of 4,304) of them had T2DM, and 10.9% had baseline heart failure (HF).8 They were then randomized 1:1 to receive dapagliflozin 10mg once daily and placebo until the first occurrence of any of the following events:8
- ≥50% decline in eGFR (Confirmed by a second serum creatinine measurement after ≥28 days)
- Onset of end-stage kidney disease (ESKD) (Defined as maintenance dialysis for ≥28 days, kidney transplantation, or an eGFR of <15mL/min/1.73m2 confirmed by a second measurement after ≥28 days)
- Death from renal or cardiovascular (CV) causes
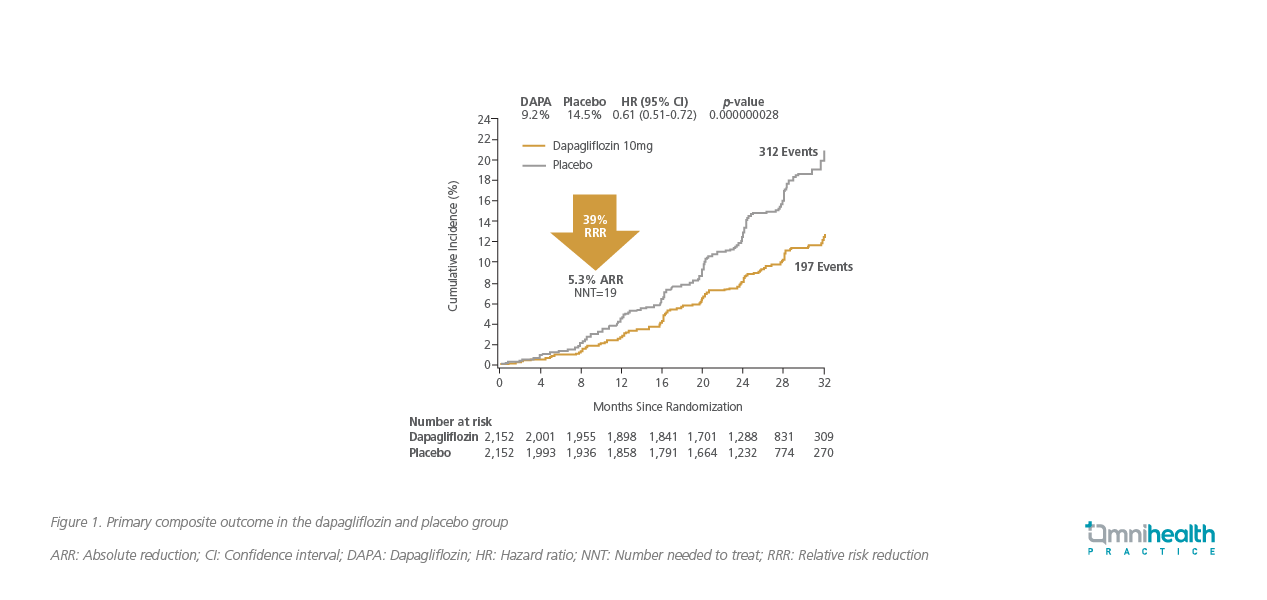
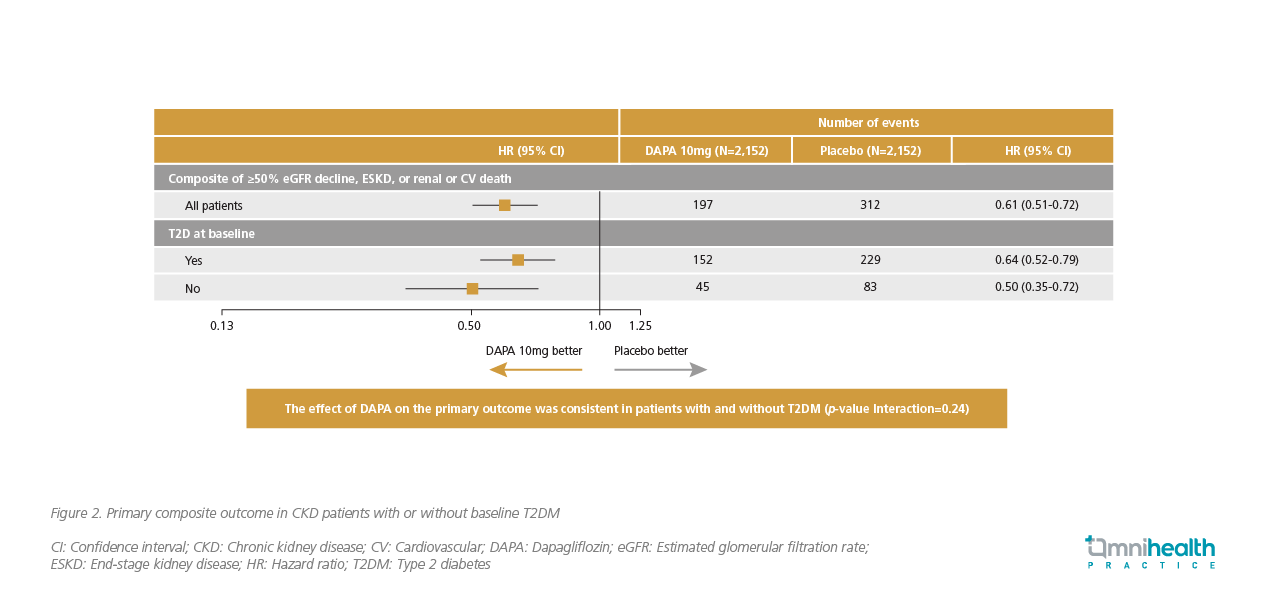
After a median follow-up of 2.4 years, the results were encouraging.8 Dapagliflozin demonstrated a 39% relative risk reduction in the primary composite endpoint (Defined as a sustained ≥50% decline in eGFR, ESKD, renal or CV deaths) compared to placebo (HR=0.61; 95% CI: 0.51-0.72, p<0.001).8 These major events occurred in 9.2% of patients in the dapagliflozin group compared with 14.5% in the placebo group (Figure 1).8 A further analysis revealed that dapagliflozin’s efficacy was consistent in patients with or without T2DM (HR=0.64; 95% CI: 0.52-0.79 vs. HR=0.50; 95% CI: 0.35-0.72, p=0.24) (Figure 2).8 For patients with baseline HF, a prespecified subgroup analysis showed that dapagliflozin reduced their risk of the primary outcome by 42%, which was comparable to those without baseline HF [HR=0.58 (95% CI: 0.37-0.91) vs. HR=0.62 (95% CI: 0.51-0.75), p=0.59].12
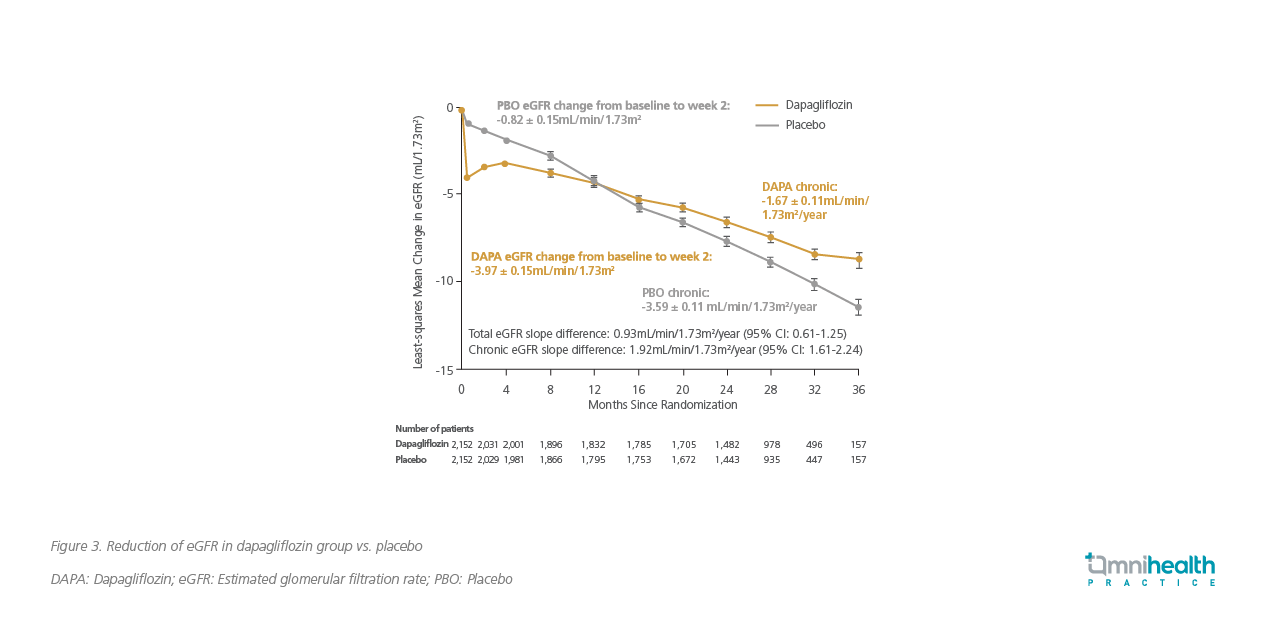
On the other hand, dapagliflozin also demonstrated a significantly slower decline in eGFR than the placebo group with a between-group difference of 1.92mL/min/1.73m2/year (Figure 3), verifying the prominent kidney-preserving effect of this SGLT2 inhibitor.8
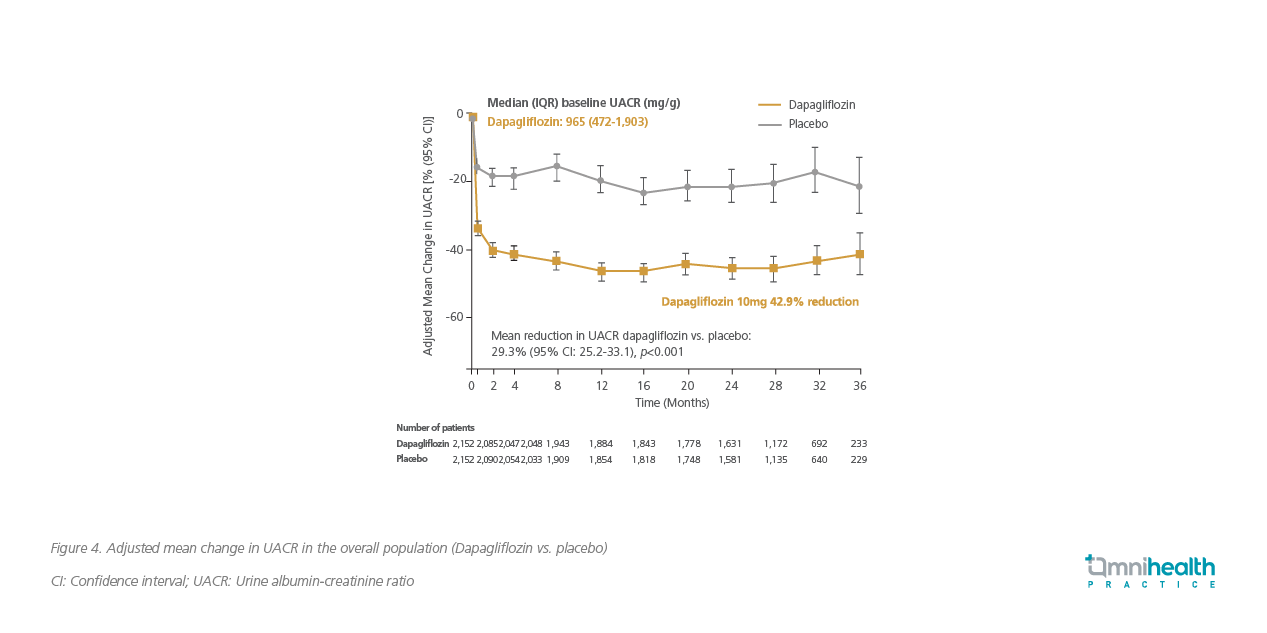
A subgroup analysis of the DAPA-CKD trial assessing albuminuria changes showed that dapagliflozin significantly reduced the geometric mean of UACR by 29.3% compared to placebo (95% CI: -33.1 to -25.2, p<0.0001) (Figure 4).13 In particular, among patients with baseline UACR ≥300mg/g, dapagliflozin was shown to increase the likelihood of UACR stage regression (HR=1.81; 95% CI: 1.60-2.05), while in patients with UACR <3,000mg/g at baseline, dapagliflozin decreased the possibility of progression in UACR stage by almost 60% (HR=0.41; 95% CI: 0.32-0.52).13
Based on the promising results of the above studies, dapagliflozin is now recommended as one of the first-line treatments by the 2020 Kidney Disease Improving Global Outcomes (KDIGO) guidelines and the American Diabetes Association (ADA) Standards of Care in Diabetes 2021 for DKD management.7,14 “Dapagliflozin is an amazing drug that should be preferably used in T2DM patients with CKD, with its primary evidence of reducing CKD progression and acceptable safety profile in the CKD population, including patients with eGFR as low as 30mL/min/1.73m2,” Prof. Cooper highlighted. Prof. Cooper further recognized dapagliflozin’s value in DKD by suggesting its use in T2DM patients even when optimal glycaemic control is achieved, purely for its reno-protective benefits.
Conclusion
The diagnosis of CKD and awareness of DKD, which is associated with significantly higher rates of morbidity and mortality, remain low. Besides, conventional treatment options such as ACEIs and ARBs failed to fulfill the unmet medical needs of the CKD and DKD patients. Therefore, routine CKD screening with both eGFR and UACR in T2DM patients, and a novel therapy such as dapagliflozin, the SGLT2 inhibitor with significant reno-protective effects beyond glucose control, are very much needed. With the superior efficacy data of dapagliflozin in delaying CKD progression, it is anticipated that dapagliflozin could help revolutionize the management of the disease, bringing added clinical benefits to patients.

