MEETING HIGHLIGHT
Dapagliflozin as a treatment reduces worsening heart failure and cardiovascular death among heart failure patients
At the 8th Asian Preventive Cardiology & Cardiac Rehabilitation Conference organized by the Hong Kong College of Cardiology, Professor Yu, Cheuk-Man, Director of Heart Centre at Hong Kong Baptist Hospital and Honorary Clinical Professor at The Chinese University of Hong Kong, discussed the role of sodium-glucose cotransporter 2 (SGLT2) inhibition in the prevention and treatment of heart failure (HF). He reviewed the results of the DAPA-HF trial, which demonstrated that dapagliflozin effects on HF as a treatment is consistent among patients with and without type 2 diabetes.
HF morbidity and mortality rates are high and have not improved despite advances in therapy
HF is a disease associated with high morbidity and mortality, and as many as 1 in 5 individuals will develop HF over their lifetime.1 HF was estimated to affect approximately 64 million people worldwide in 2016, although this is likely to be an underestimate particularly in Asian populations.2
HF morbidity and mortality rates are high despite advances in therapy, with approximately half of US HF patients dying within 5 years of their diagnosis.3,4 HF mortality risk is similar to that of some common cancers in both men and women, and survival is worse than that for prostate cancer in men and breast cancer in women.5 In fact, from 1988-2015, mortality improvements in the US have been seen in major cardiovascular disease (CVD) and atherosclerosis but not for HF nor arrhythmia.6
Dapagliflozin provides an alternative in the HF treatment landscape
Currently, there are gaps in the use and dosing of drugs recommended by guidelines for the treatment of HF with reduced ejection fraction (HFrEF). From the US cardiology practices in the Change the Management of Patients with HF (CHAMP-HF) registry, a substantial proportion of HF patients were not treated despite having no contraindications to HF medications.7 Among those eligible, 26.2% were not prescribed of angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blocker (ARB) or angiotensin receptor-neprilysin inhibitor (ARNI) therapy.7 At the same time, 32.9% and 65.9% of eligible patients were not prescribed of beta-blocker therapy and mineralocorticoid receptor antagonists (MRA), respectively.7 Even when guideline-directed medical therapy was used, only 17.5%, 14% and 27.5% of patients have received target doses of ACEis/ARBs, ARNI and beta-blockers, respectively.7 In this regard, SGLT2 inhibitors such as dapagliflozin can provide an additional option for undertreated HF patients.
Positive results for dapagliflozin in the DECLARE-TIMI 58 trial led to its investigation in the DAPA-HF study
The results of the DECLARE-TIMI 58 trial, which included 17,160 patients with type 2 diabetes and established atherosclerotic CVD or multiple risk factors for atherosclerotic CVD, showed that dapagliflozin resulted in a significantly lower rate of cardiovascular death or hospitalization for HF when compared to placebo after a median follow-up of 4.2 years (4.9% vs. 5.8%; HR=0.83; 95% CI: 0.73-0.95; p=0.005).8 In a further meta-analysis of SGLT2 inhibitor cardiovascular outcome trials, it was revealed that dapagliflozin has a consistent benefit in reducing the risk of hospitalization for HF in both patients that have atherosclerotic CVD (HR=0.83; 95% CI: 0.71-0.98) and those without (HR=0.84; 95%CI: 0.67-1.04).9 As 90% of the patients in the DECLARE-TIMI 58 trial did not have a history of HF at baseline, the treatment with dapagliflozin appeared to have a benefit in preventing new clinical HF from occurring among patients with type 2 diabetes.8
Dapagliflozin showed significant reductions in hospitalization for HF and death in patients with and without type 2 diabetes in the DAPA-HF trial
To further investigate the cardiovascular outcomes of SGLT2 inhibitors in patients without type 2 diabetes, the DAPA-HF trial has enrolled 4,744 patients who had symptomatic HFrEF for ≥2 months, left ventricular ejection fraction (LVEF) ≤40% within the past year and estimated glomerular filtration rate (eGFR) ≥30ml/min/1.73m.10 Unlike the DECLARE-TIMI 58 trial, which included only patients with type 2 diabetes, 55% of the patients in DAPA-HF did not have type 2 diabetes.10 Patients were randomized to either dapagliflozin 10mg or placebo, and both treatment groups had similar HF characteristics and were receiving recommended HFrEF therapy.10 Patients were followed for a mean of 18.2 months.10 The primary endpoint was a composite of worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or cardiovascular death.
Compared to placebo, dapagliflozin significantly reduced the primary endpoint by 26% (HR=0.74; 95% CI: 0.65-0.85, p<0.001), with an absolute risk reduction of 4.9% (Figure 1) and a significant beneficial effect seen as early as day 28 (p=0.03).10 The relative risk reduction (RRR) in the primary composite outcome was similar between patients with and without type 2 diabetes (25% vs. 27%).10
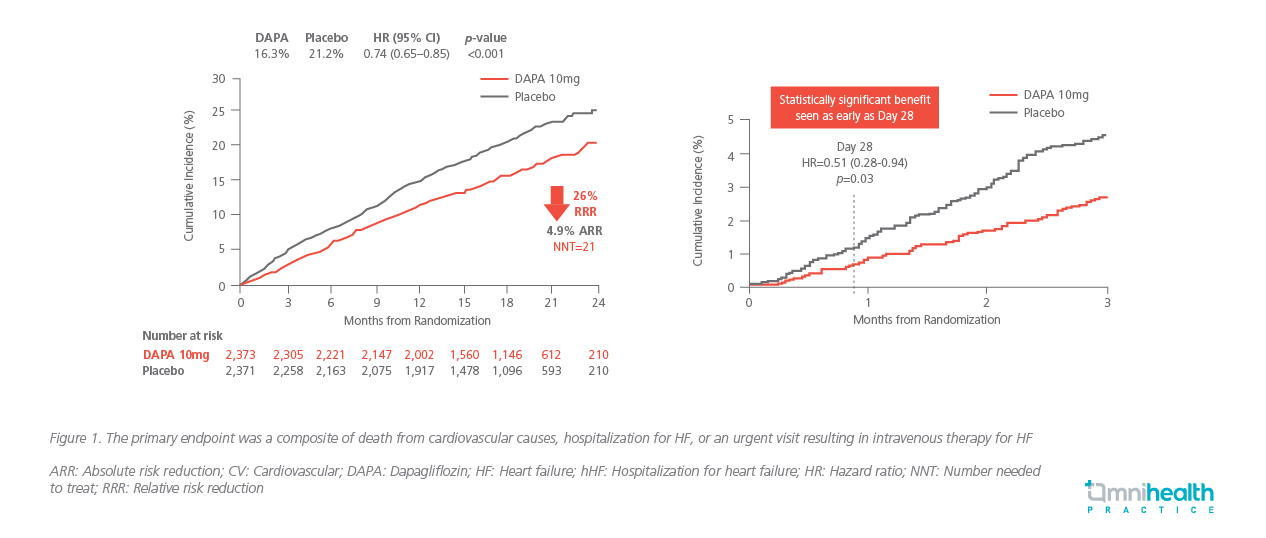
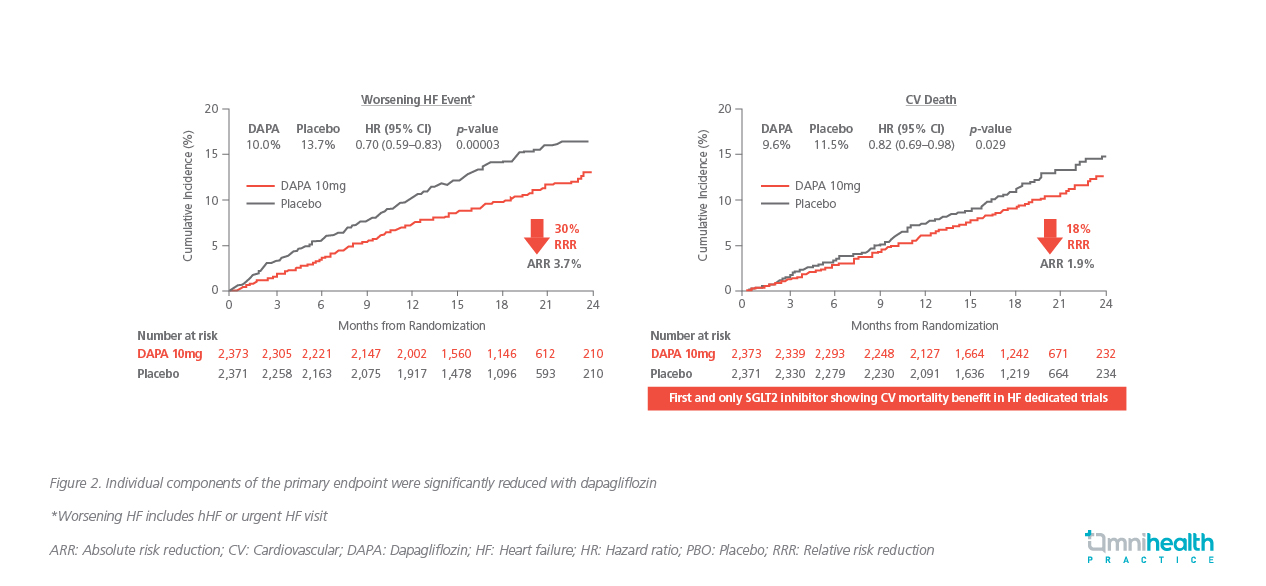
Furthermore, when analyzed separately, the individual components of the composite endpoint were significantly reduced with dapagliflozin (30% RRR in hospitalization for HF or urgent HF visits, 18% RRR for cardiovascular death) (Figure 2).10 A key secondary endpoint was death from any cause, which was significantly reduced by 17% (HR=0.83; 95% CI: 0.71-0.97) with dapagliflozin compared to placebo.10
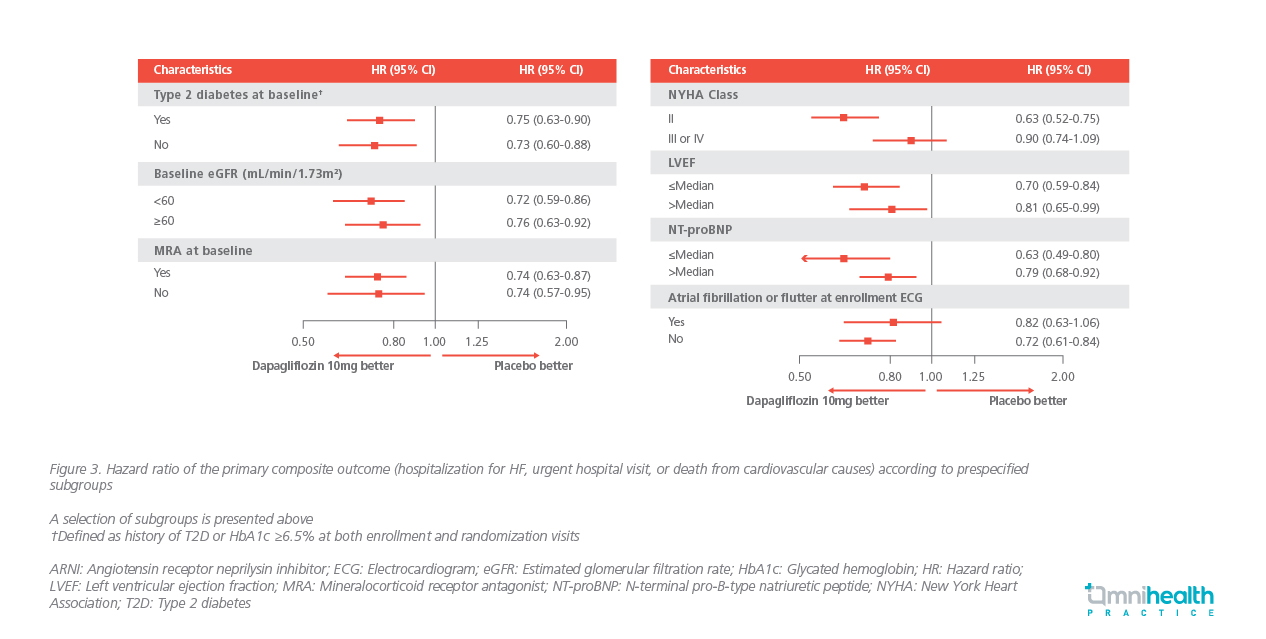
A consistent treatment benefit was seen across a number of subgroups in a prespecified subgroup analysis, including baseline eGFR, New York Heart Association (NYHA) functional class, LVEF category, N-terminal pro-B-type natriuretic peptide (NT-proBNP) level and atrial fibrillation or flutter at enrollment ECG (Figure 3).10
The beneficial effect of dapagliflozin was also consistent regardless of the HF therapy choice or dose; in a recently published DAPA-HF sub-analysis, dapagliflozin has consistently reduced the risk of worsening HF and death in HFrEF patients regardless of previous treatment with the combination of renin-angiotensin system (RAS) blocker (ACEi/ARB), beta-blocker and MRA, or were not on all three of these treatments at baseline.11 In the same sub-analysis, the benefit of dapagliflozin was shown to be consistent across a range of LVEF from <26% to >35%, as well as the time of measurement of LVEF at ≤6 or >6 months prior to patient randomization.12
In terms of symptoms relief, patients receiving dapagliflozin were 18% more likely to have symptom benefit as measured by improvement in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score (KCCQ-TSS) compared to placebo.10 The KCCQ is a 23-item, selfadministered disease-specific survey that quantifies symptoms, physical function, quality of life and social function over the previous 2 weeks; the TSS quantifies symptom frequency and severity.13 In other studies, dapagliflozin was also shown to have more common symptom improvement and less frequent symptom deterioration when compared to placebo.11,13
In terms of safety, dapagliflozin has a favorable side effect profile with safety outcomes being similar between dapagliflozin and placebo, irrespective of ARNI treatment or the presence or absence of diabetes.10,11 The frequency of adverse events related to volume depletion, renal dysfunction and hypoglycemia did not differ between treatment groups.10 In addition, more patients in the dapagliflozin group were able to decrease their dose of diuretic therapy and fewer required an increase in diuretic dose at 6 and 12 months.14
Dapagliflozin in the new proposed therapeutic standard of HFrEF
May 2020, dapagliflozin became the first SGLT2 inhibitor to be approved for the treatment of adults with HFrEF in the US.15 Shortly after, dapagliflozin was also approved in the European Union (EU) in November 2020 for HF patients who have symptoms of HFrEF.16 Furthermore, the Canadian Cardiac Society (CCS)/Canadian Heart Failure Society (CHFS) and European Society of Cardiology (ESC) position statement now recommends SGLT2 inhibitors to be used in HF patients, with or without diabetes, with a reduced ejection fraction (LVEF ≤40%).17,18 As HFrEF can be improved through multiple mechanistic pathways including the modulation of angiotensin II, norepinephrine, aldosterone, neprilysin and SGLT2, a comprehensive combination of ARNI, beta-blockers, MRAs and the addition of SGLT2 inhibitors should now be considered as a new therapeutic standard for HFrEF treatment (Figure 4).19,20 By modulating all mechanistic pathways early, the addition of SGLT2 inhibitors can help mediate the high risks of HFrEF and provide a new option to delay clinical progression and extend disease-free survival.20
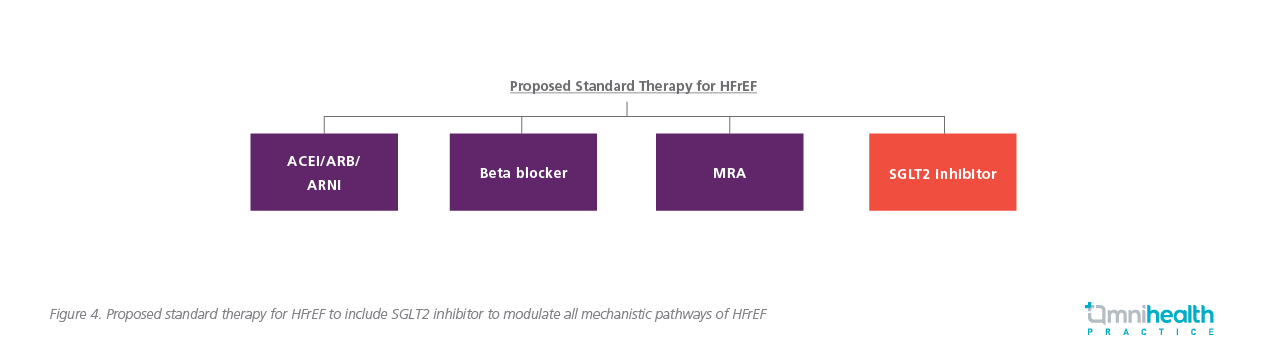
Conclusion
Findings from the DAPA-HF trial support the use of SGLT2 inhibitor dapagliflozin as the new standard of care for patients with HFrEF, with and without type 2 diabetes. Dapagliflozin has been shown to reduce HF symptoms, worsening HF, CVD death and all-cause death with a manageable safety profile, and its benefit was consistent among prespecified subgroups. In fact, dapagliflozin is the only SGLT2 inhibitor that has demonstrated a reduction in hospitalization for HF and a significant reduction in mortality as a HF treatment among patients with or without diabetes. Prof. Yu concluded, “In the pillars of therapy of HF, perhaps apart from the use of ACEi/ARB/ARNI, then subsequently also beta-blockers, MRAs, we also have the choice of SGLT2 inhibitors as a promising therapy”.

