CASE REVIEW
Dapagliflozin as the first step to heart failure quadruple therapy: Sharing of local cases
Heart failure (HF) is a life-debilitating condition affecting 26 million people worldwide.1 In Hong Kong, cardiovascular diseases represent the third major cause of mortality and account for 13.2% of all deaths in 2015.2 Recently, the sodium-glucose cotransporter 2 inhibitors (SGLT2i) dapagliflozin was approved as the treatment of HF with reduced ejection fraction (HFrEF).3,4 In 2021, the American College of Cardiology (ACC) has updated their expert consensus and now includes SGLT2i as a component of first-line quadruple treatment for patients with HFrEF.5 In a recent interview with Omnihealth Practice, Dr. Leung, Tat-Chi Godwin, Specialist in Cardiology, shared his experience in treating HFrEF with dapagliflozin and highlighted the synergistic effect of initiating all four drug classes of HFrEF treatment within 4 weeks of clinical encounter.
Background
Conventional clinical management of HFrEF relies on β-blockers in combination with renin-angiotensin-aldosterone inhibitors (RAASi) and mineralocorticoid receptor antagonist (MRA).6 This triple therapy has long been considered the standard guidelinedirected medical therapy (GDMT) for HFrEF patients.5,6 Recent findings from the phase 3 DAPA-HF study suggested the use of SGLT2i could significantly reduce the risk of worsening HF or cardiovascular death in patients with New York Heart Association (NYHA) class II to IV HFrEF when compared to placebo (16.3% vs. 21.2%; HR=0.74; 95% CI: 0.65-0.85; p<0.001).7 In particular, dapagliflozin had significantly reduced the cumulative risk of cardiovascular deaths (HR=0.82; 95% CI: 0.69-0.98; p=0.029) (Figure 1).7
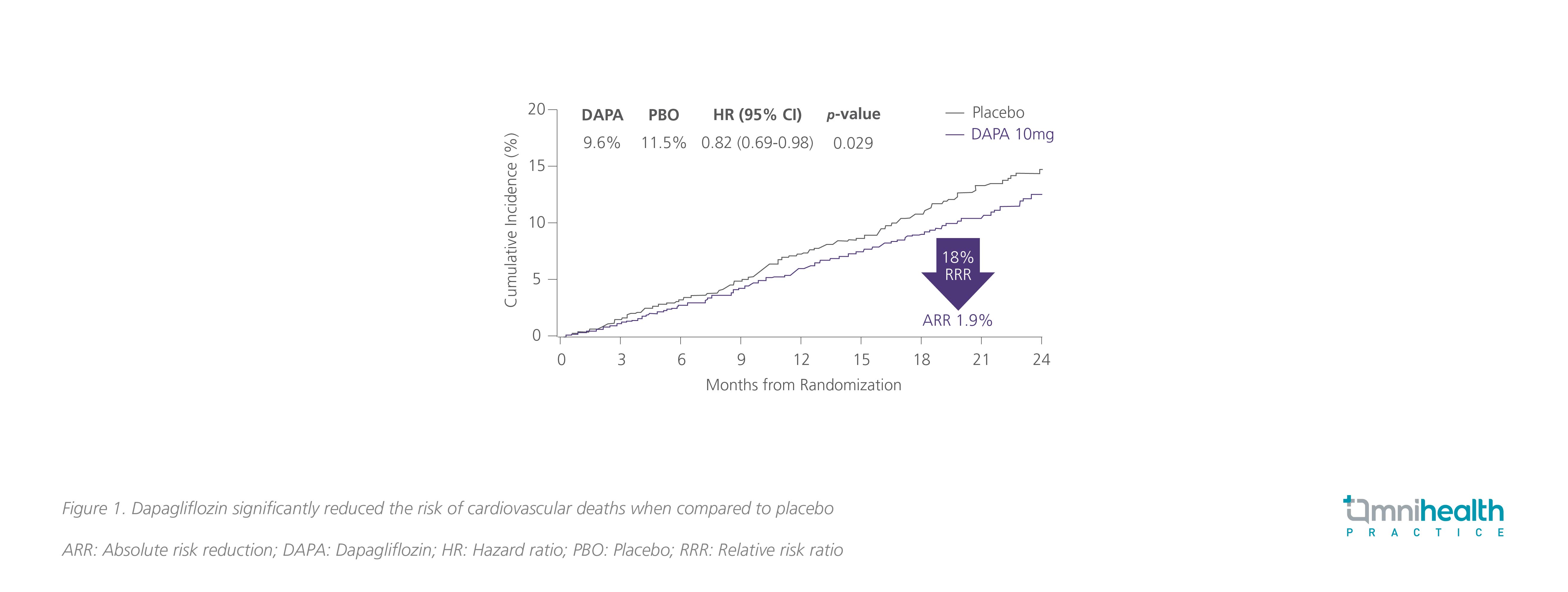
Where the DECLARE-TIMI 58 study demonstrated that dapagliflozin was able to significantly lower the rate of cardiovascular death or HF-related hospitalization among diabetic patients (4.9% vs. 5.8%; HR=0.83; 95% CI: 0.73-0.95; p=0.005), the subsequent DAPA-HF study demonstrated that this efficacy of dapagliflozin was consistent regardless of diabetic status (pinteraction=0.80), age (pinteraction=0.76) and concurrent medications including sacubitril/valsartan, diuretic dose and glucose-lowering therapy use (pinteraction=1.00, 0.61 and 0.39, respectively).8-13 Notably, the benefit and safety of dapagliflozin were consistent across a wide spectrum of age groups from 22 to 94-years-old, indicating that advanced age does not justify withholding dapagliflozin in HFrEF patients.10 Where there is a stepwise increase of risk for cardiovascular death or worsening HF in accordance with the timing of the most recent HF hospitalization, dapagliflozin had the greatest absolute risk reductions in patients with a more recent HF hospitalization at 12 or fewer months ago (p=0.05).14
As the only SGLT2i with demonstrated mortality and morbidity benefit in HFrEF patients, dapagliflozin was approved by the Food and Drug Administration and the European Medicines Agency in May and November 2020, respectively for HFrEF.3,4 As the disease course of HFrEF can be modified by concurrently targeting RAAS, sympathetic nervous system, neprilysin and SGLT2, the 2021 ACC updated expert consensus now includes SGLT2i as a component of first-line quadruple treatment for patients with HFrEF.5,15 With a comprehensive combination of SGLT2i, β-blockers, RAASi and MRA, the quadruple therapy can incur a maximum survival benefit for HFrEF patients through the independent mechanistic pathway of each drug class.
Case report
In clinical practice, a similar survival benefit to DAPA-HF can be observed among local patients receiving dapagliflozin.
Case 1:
In a local case of an 80-year-old lady with known hyperlipidemia, impaired fasting blood glucose and mild renal impairment with an estimated glomerular filtration rate (eGFR) of 40-50mL/min/1.73m2, she was presented with anterior myocardial infarction and received percutaneous coronary intervention (PCI) with stent implanted to the proximal left anterior descending artery. In the 6 months following myocardial infarction, she was recurrently hospitalized for decompensated HF. Echocardiography showed an ejection fraction of 35%, and she was diagnosed with NYHA class III HFrEF.
Initially, she was given angiotensin receptor-neprilysin inhibitor (ARNI) to manage HFrEF, but she could not tolerate the treatment due to hypotension, postural dizziness and hyperkalemia. She then switched to β-blockers but could only tolerate a low-dose due to hypotension, malaise and cold extremities. Based on the DECLARE-TIMI 58 study, 10mg/day dapagliflozin was prescribed.
Within 6 months of dapagliflozin treatment, her HF symptoms had greatly improved and her NYHA status was improved to class II. Notably, her blood pressure remained stable throughout treatment and she can now tolerate low-dose ARNI and higher dose β-blockers which can be attributed to the renal protective and cardiac beneficial effects of dapagliflozin. In the following year, she was not hospitalized and enjoyed a much-improved quality of life.
Case 2:
In another local case of a 50-year-old gentleman, he was newly diagnosed with HF caused by dilated cardiomyopathy. Except borderline hypertension, he had good past health and normal renal function. Before he was hospitalized, he had progressive shortness of breath on exertion, orthopnoea and decreased exercise tolerance over a few weeks. He was then admitted for HF shortly after.
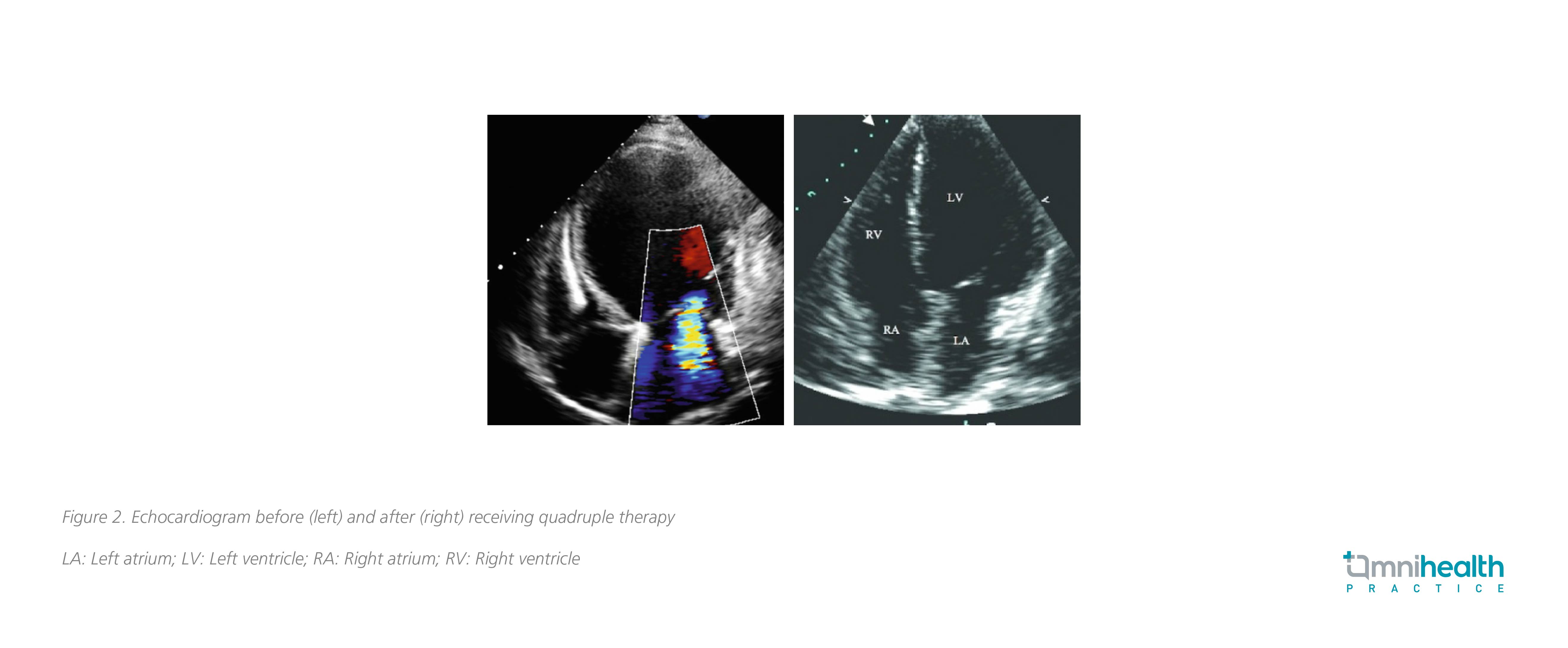
Echocardiogram showed a reduced ejection fraction of 25-30% with a significantly dilated left ventricle (LV). Moderate functional mitral regurgitation was observed, and computed tomography (CT) coronary angiogram showed normal coronary arteries. Initially, he was given intravenous furosemide and his HF symptoms were partially improved. With reference to the results of DAPA-HF which dapagliflozin showed a significant reduction of cardiovascular death or HF-related hospitalization among HFrEF patients, she was given a combination of low-dose ARNI, β-blocker, MRA and 10mg dapagliflozin daily during his hospital stay. The quadruple treatment was well-tolerated with no significant decrease in blood pressure.
With symptoms under control and NYHA status improved to class I/II, he was discharged after 1 week of hospitalization. ARNI and β-blockers were then titrated to the maximum target dose during follow-up. Then 4 months later, echocardiogram showed a decreased LV size with ejection fraction improved to 45%, suggesting that quadruple treatment including dapagliflozin may improve LV remodeling (Figure 2).
Discussion
In practice, one common challenge in treating HF is to overcome clinical inertia.16 In particular to patients who achieved stable disease, clinicians may be hesitant or unable to prescribe GDMT or up-titrate existing HF treatment to the target dose in fear of potential adverse effects.16 Indeed, hyperkalemia is a common side effect among patients with severe renal dysfunction, and these patients are often unable to tolerate angiotensin-convertingenzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) and MRA.16 Moreover, β-blockers are contraindicated for those with asthma and bradyarrhythmia.16 In the real-world setting, the CHAMP-HF registry revealed that 26.2%, 32.9% and 65.9% of eligible patients were not prescribed with ARNI/ACEi/ARB, β-blockers and MRA, respectively, despite having no contraindications.17 A substantial proportion of these patients also did not reach the guideline-recommended target dose.17
Whereas these HF treatments may be limited by concerns on adverse effects, dapagliflozin has a mild side effect profile and a simple dosing regimen of one pill per day with no dose titration that can help clinicians overcome clinical inertia. In the DAPA-HF study, dapagliflozin was well tolerated and had a similar rate of serious adverse events related to volume depletion (1.2% vs. 1.7%; p=0.23) and a lower risk of serious renal adverse events (1.6% vs. 2.7%; p=0.009) when compared to placebo.7 From clinical experience, SGLT2i is associated with diuretic effect and the early prescription of dapagliflozin can help reduce the need of loop diuretics particularly among patients with acute decompensated HF. As exemplified in both shared cases, dapagliflozin is well-tolerated and can be initiated after HFrEF is confirmed by echocardiogram. Particularly, the addition of dapagliflozin in Case 1 improved the treatment tolerance to other drug classes and enabled concurrent GDMT to leverage its early survival benefit.
Although it might be assumed that patients with stable HFrEF would have little to gain from the additional therapy, a post-hoc analysis showed that the benefit of dapagliflozin was consistent across HF duration of ≥2 to ≤12 months, >1 to 2 years, >2 to 5 years and >5 years (pinteraction=0.26).18 In fact, the greatest absolute benefit was observed among those with the longest duration of HF (18 months for HF >5 years vs. 28 months for HF ≥2 to ≤12 months).18 With a well-demonstrated safety and efficacy profile, dapagliflozin can be the key to overcome clinical inertia and enable guideline-recommended quadruple therapy.
A new HF treatment sequence to consider
As the magnitude of treatment benefit of each drug class is independent, it is clinically important to prescribe all available drug classes even at lower starting doses to maximize the potential survival benefit.19 In earlier practice, clinicians typically begin HFrEF treatment in sequence by which HF drug classes were developed.19 Not only does this sequence assume that these treatment options are only effective when titrated to the target dose, but it would also require over 6 months before all recommended options are prescribed.19
Considering that the benefit of each HFrEF medication is detectable in 30 days, all four components of the quadruple therapy should be initiated within 4 weeks for achieving optimal clinical outcome.19 To achieve this target, the addition of new drug classes should be prioritized over the up-titration of existing drug classes.19 The sequencing of drug classes should also be aimed at improving the safety and tolerability of subsequent treatment options; SGLT2i and ARNI can improve renal function and potassium homeostasis, thus improving the tolerability towards MRAs.19
Step 1: SGLT2i such as dapagliflozin should be considered in step 1 of the quadruple treatment along with β-blockers once HFrEF is confirmed (Figure 3).19 In addition to having an identical starting and target dose that require no up-titration, SGLT2i has a low side effect profile and can be safely prescribed to those with hypotension, renal dysfunction or hyperkalemia.19 However, it should be noted that β-blockers are associated with fluid retention, fatigue and bradycardia, and close monitoring should be given when prescribed. According to Dr. Leung, SGLT2i may also be preferable to ARNI for symptom control in practice due to its once-daily dosing and cost-effectiveness.
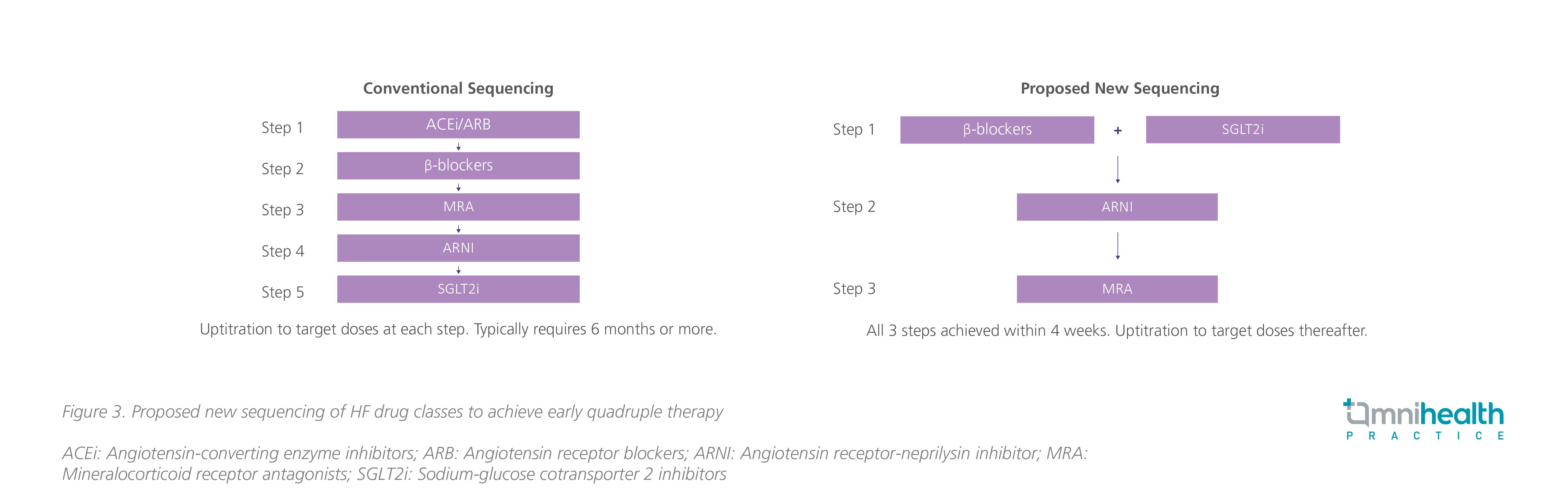
Step 2: ARNI should be considered within 1-2 weeks of initiating SGLT2i and β-blocker. For patients at risk of hypotension, ARB may be considered before switching to ARNI.19
Step 3: MRA should be considered within 1-2 weeks after initiating ARNI/ARB treatment, if serum potassium is normal and renal function is not severely impaired.19
With this new approach, quadruple therapy should be achievable within 4 weeks of treatment. Again, it is important to prioritize the prescription of all 4 drug classes to leverage the independent mechanistic benefit of HFrEF treatments over the up-titration of existing drug classes. “The SGLT2i dapagliflozin is a convenient and well-tolerated option with good side effect profile and can be considered as the first step of the quadruple HF therapy to maximize patient outcome,” concluded Dr. Leung.
Conclusion
To maximize the HF treatment benefit, guideline-recommended quadruple therapy should be prescribed as early as possible to leverage the independent mechanistic benefit of available drug classes. With the ACC expert consensus now recommending the combination of ARNI/ACEi/ARB, β-blocker, MRA, and SGLT2i as first-line HFrEF treatment, it is important to prioritize the prescription of all 4 drug classes over the up-titration of existing options. Given its safety and efficacy, dapagliflozin along with β-blockers should be given in step 1 of the HFrEF first-line treatment followed by ARNI/ACEi/ARB and MRA to achieve guideline-recommended quadruple therapy within 4 weeks of treatment initiation.

