CONFERENCE UPDATE: ACC 2024
“Inclisiran first” strategy lowers LDL-C in real-world ASCVD patients: Results from the VICTORION-INITIATE trial
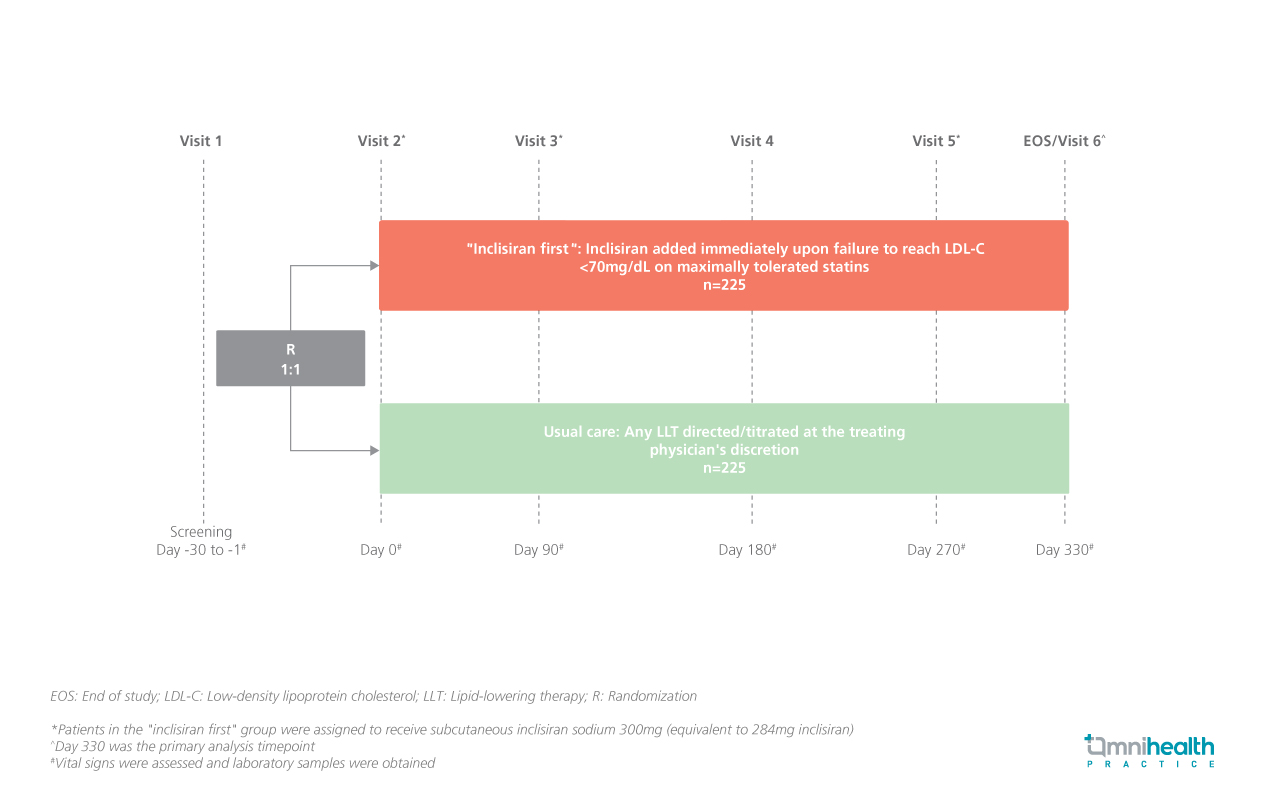
STUDY DESIGN
A reduction in low-density lipoprotein-cholesterol (LDL-C) is associated with improvements in cardiovascular (CV) outcomes in patients with atherosclerotic cardiovascular disease (ASCVD).1 As such, multiple guidelines recommend LDL-C targets of <55mg/dL and <70mg/dL based on baseline risks.1 However, many patients fail their LDL-C goals due to clinical inertia, medication nonadherence, side effects, and/or poor healthcare access.1 Inclisiran is a first-in-class small interfering RNA (siRNA) that inhibits hepatic proprotein convertase subtilisin/kexin type 9 (PCSK9) production and increases LDL receptor expression and LDL-C clearance.1 In clinical studies, twice-yearly administration of inclisiran has been shown to achieve a 50% reduction in LDL-C.1
The VICTORION-INITIATE study is a prospective, randomized, open-label, phase 3b trial conducted to evaluate an “inclisiran first” implementation strategy compared to usual care in patients with ASCVD in representative US clinical settings.1 The study included adult patients with a history of ASCVD, LDL-C levels ≥70mg/dL or non-high density lipoprotein-cholesterol (non-HDL-C) levels ≥100mg/dL and fasting triglycerides <500mg/dL who were treated with maximally tolerated statin therapy or had documented statin intolerance.1 Participants were randomized 1:1 to receive “inclisiran first” (n=225) or the usual care (n=225).1 The “inclisiran first” implementation strategy is the immediate addition of inclisiran upon failure to achieve an LDL-C of <70mg/dL despite maximally tolerated statin therapy.1
The primary endpoints of the study were the percent change in LDL-C from baseline to day 330 and the discontinuation of statin (defined as no statin use ≥30 days before the end-of-study visit).1 Key secondary endpoints included the proportion of patients achieving the pre-specified LDL-C goals at day 330 and changes in background lipid-lowering therapy (LLT).1 A safety analysis was also performed at each follow-up visit.1
FINDINGS
|
Primary endpoints: |
|
|
|
|
Secondary endpoints: |
|
|
|
|
|
|
Safety: |
| The safety profile of the “inclisiran first” implementation strategy was comparable to usual care1 |
| 62.8% and 53.7% of the “inclisiran first” group and the usual care group experienced ≥1 treatment-emergent adverse event (TEAE), respectively1 |
| Injection-site TEAEs were more common in the “inclisiran first” group than the usual care group (10.3% vs. 0.0%), where almost all cases were mild with only 1 case of moderate injection-site TEAE1 |
"Results from VICTORION-INITIATE trial demonstrate the clinical value of initiating inclisiran earlier in the treatment pathway"
Dr. Michael J. Koren
Department of Cardiology,
Jacksonville Center for Clinical Research,
Jacksonville, United States

