CONFERENCE UPDATE: ACC 2024
LDL-C reduction with lerodalcibep in patients at very high or high risk for CVD: The phase 3 LIBerate-HR study
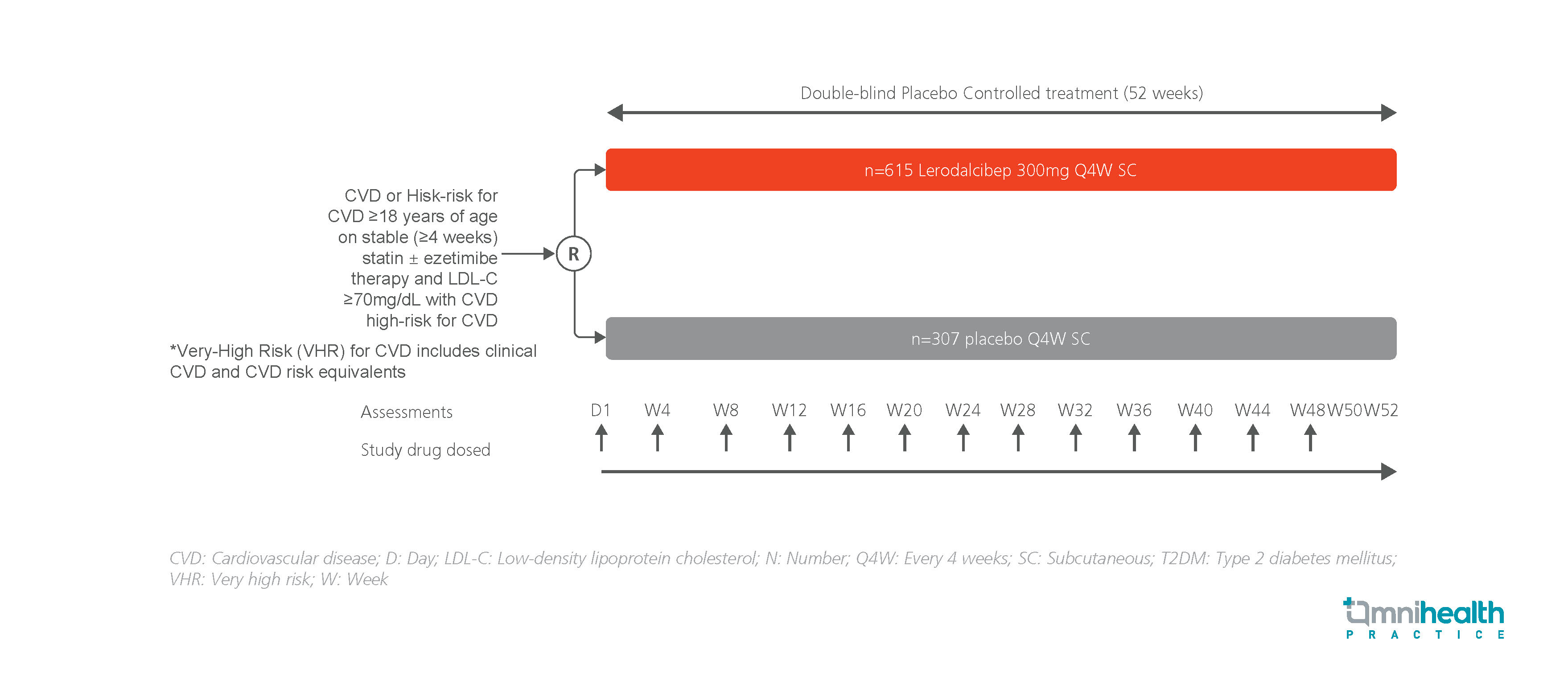
STUDY DESIGN
Lerodalcibep is a third-generation proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitor that prevents PCSK9 from binding to low-density lipoprotein receptors (LDLRs).1 Low-density lipoprotein-cholesterol (LDL-C) clearance from the bloodstream is enhanced as a result of LDLR degradation being blocked.1 Compared to monoclonal antibodies (mAbs), lerodalcibep is smaller in size and has a higher solubility, enabling prolonged and stable LDL-C reductions.1 In a prior phase 2 trial, lerodalcibep at a dose of 300mg monthly was shown to be effective in lowering LDL-C by >70%.1
The LIBerate-HR is a phase 3 study conducted to assess the efficacy and safety of lerodalcibep in patients at very high or high risk for CVD.1 The study included 922 adult patients with cardiovascular disease (CVD) or at high risk for CVD, who were receiving stable doses of statin with or without ezetimibe and have LDL-C levels ≥70mg/dL and CVD or LDL-C levels ≥100mg/dL and a high risk of CVD.1 Patients with very high risk, including those with clinical CVD or CVD risk equivalents were also included.1 They were randomized (2:1) to receive monthly subcutaneous (SC) injections of lerodalcibep 300mg (n=615) or a matching placebo (n=307) for 52 weeks.1
The coprimary endpoints were the percent change in LDL-C levels from baseline to week 52 and the mean change in LDL-C levels of weeks 50 and 52.1 Key secondary outcomes included the percentage of patients achieving ≥50% LDL-C reduction and LDL-C targets as well as the changes in other lipid and apolipoproteins.1
| Co-primary efficacy endpoints | Secondary outcomes |
| Percentage change from baseline in LDL-C at week 52 and the mean change of weeks 50 and 52 | Achievement of LDL-C targets, other lipid and apolipoprotein changes |
FINDINGS
|
Primary endpoints: |
|
|
Secondary endpoints: |
|
|
Safety: |
|
“Lerodalcibep offers a novel, effective alternative to existing PCSK9 inhibitors with substantial LDL-C reductions on top of existing oral agents”
Dr. Eric Klug
Division of Cardiology,
Faculty of Health Sciences,
University of the Witwatersrand,
Johannesburg, South Africa
A case review on cardiac risk stratification in the secondary prevention of cardiovascular diseases
Cardiac risk stratification is an assessment used to evaluate a patient's risk of developing cardiovascular disease (CVD) or the risk of a cardiac event occurring in noncardiac surgeries. CVDs are the number one cause of death

