CONFERENCE UPDATE: ACC 2024
Triglyceride reduction with olezarsen in HTG patients with high CV risk: The BRIDGE-TIMI 73a trial
STUDY DESIGN
Triglyceride-rich lipoproteins (TRLs) are atherogenic and increase cardiovascular (CV) risk comparable with low-density lipoprotein (LDL).1 Their clearance is facilitated by lipoprotein lipase (LPL) which hydrolyses the triglyceride (TG) component.1 However, some TRLs express apolipoprotein C-III (APOC3) which inhibits LPL and slows TRL clearance.1 Loss-of-function variants in APOC3 have been associated with lower triglyceride levels and reduced CV risk.1 Olezarsen is a GalNAc3-conjugated antisense oligonucleotide that targets the APOC3 mRNA.1
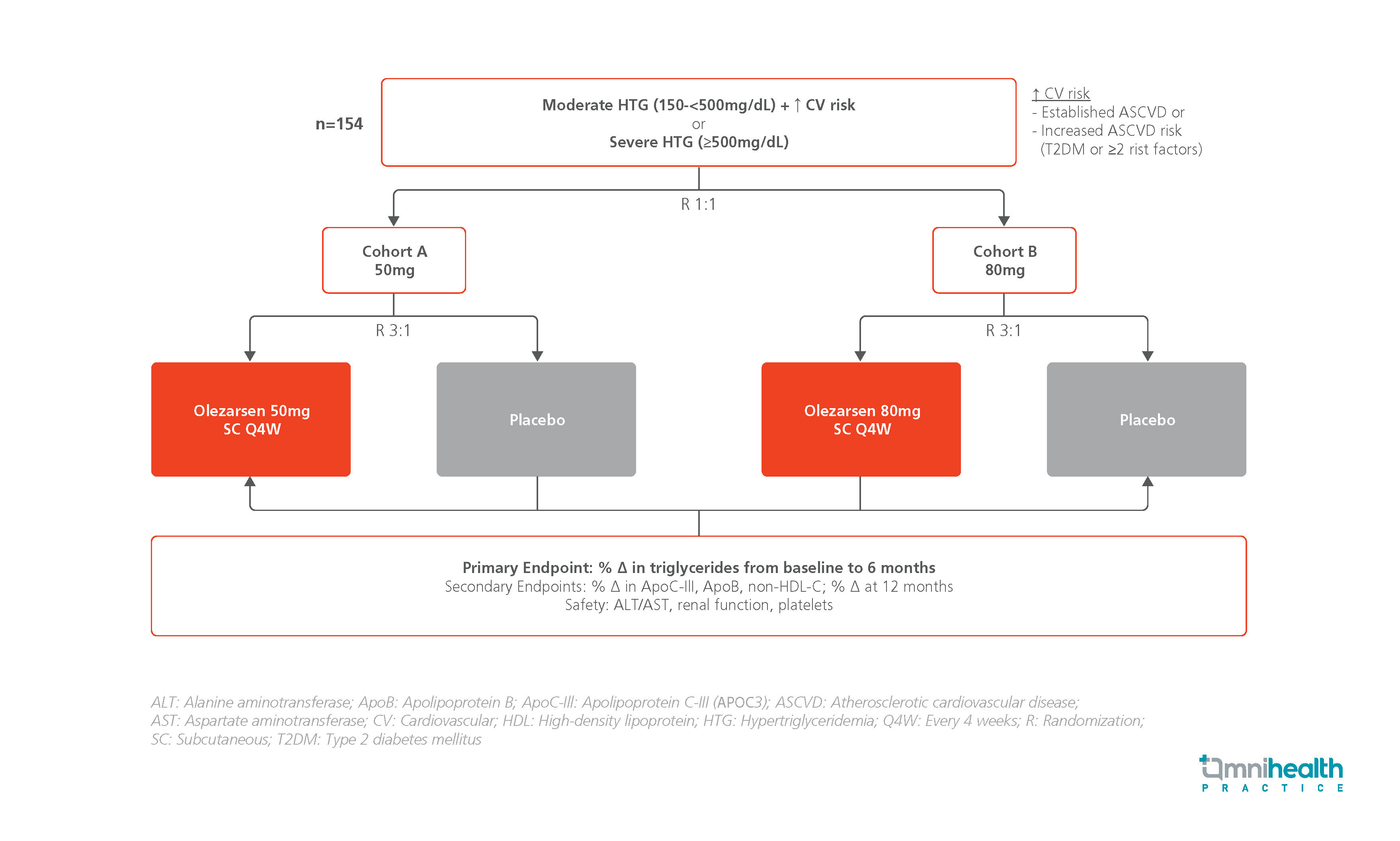
The BRIDGE-TIMI 73a study is a phase 3 trial which evaluated the efficacy and safety of olezarsen in patients with moderate hypertriglyceridemia (HTG) and elevated CV risk or with severe HTG.1 The study included 154 patients with TG levels between 150mg/dL and <500mg/dL with a high CV risk or with TG levels ≥500mg/dL.1 A high CV risk was defined as the presence of established atherosclerotic CV disease (ASCVD) or increased ASCVD risk (presence of type 2 diabetes mellitus [T2DM] or ≥2 risk factors).1 They were randomized 1:1 into 2 cohorts, each receiving a different dose of olezarsen.1 Patients in cohort A were further randomized 3:1 to receive olezarsen 50mg subcutaneously (SC) every 4 weeks (Q4W) or placebo.1 Similarly, patients in cohort B were randomized (3:1) to receive olezarsen 80mg SC Q4W or placebo.1
The primary endpoint was the percentage change in TG from baseline to 6 months.1 Key secondary endpoints included the percentage change in APOC3, ApoB and non-high-density lipoprotein cholesterol (non-HDL-C) from baseline to 12 months.1
FINDINGS
|
Primary endpoints: |
|
|
Secondary endpoints: |
|
|
Safety: |
|
“Olezarsen led to meaningful reductions in apolipoprotein B and non-HDL-C, markers of atherogenic risk”
Dr. Brian Bergmark
Brigham and Women's Hospital
Boston, Massachusetts,United States

