CONFERENCE UPDATES: ESC 2025
Vericiguat improves survival and CV outcomes across broad HFrEF spectrum: Results from the VICTOR and VICTORIA pooled analysis
STUDY DESIGN
Vericiguat has been investigated in two large phase 3 trials, VICTOR and VICTORIA, in patients with heart failure with reduced ejection fraction (HFrEF).1 In VICTORIA, vericiguat reduced the risk of cardiovascular (CV) death or hospitalization for heart failure (HHF) in patients with recent worsening HF.1 Whereas in VICTOR, vericiguat reduced CV death, sudden cardiac death, HF-related death, and all-cause mortality but did not lower the composite endpoint of CV death or HHF.1 In VICTORIA, the benefit was attenuated in patients with very high NT-proBNP levels (>5,314pg/mL), supporting the 6,000pg/mL cutoff used in VICTOR trial design.1
These findings supported a pooled analysis of VICTOR and VICTORIA to comprehensively evaluate treatment outcomes.1 The individual patient-level prespecified pooled analysis reported the efficacy and safety of vericiguat on both the composite endpoint of CV death or first HHF and its individual components (CV death and first HHF), in addition to all-cause mortality and treatment effects across major prespecified subgroups.1
VICTORIA enrolled patients with HFrEF, defined by left ventricular ejection fraction (LVEF) <45% within 12 months, congestive heart failure (CHF) NYHA class II-IV, a recent HF hospitalization or intravenous diuretic use and NT-proBNP >1,000pg/mL.1 VICTOR, by contrast, included ambulatory patients with HFrEF and LVEF <40% within 12 months but without recent HF events, requiring NT-proBNP levels between 600pg/mL-6,000pg/mL, stable non-decompensated status with no recent changes in guideline-directed medical therapy (GDMT).1
In total, 11,155 participants meeting these criteria were randomized 1:1 to receive vericiguat (n=5,579) or placebo (n=5,576), on top of standard GDMT.1 Baseline characteristics and background medical therapies were well-balanced between treatment arms.1 The primary endpoint of the pooled analysis was time to CV death or first HHF.1 Secondary endpoints included time to CV death, time to first HHF, total HHF events and all-cause mortality.1
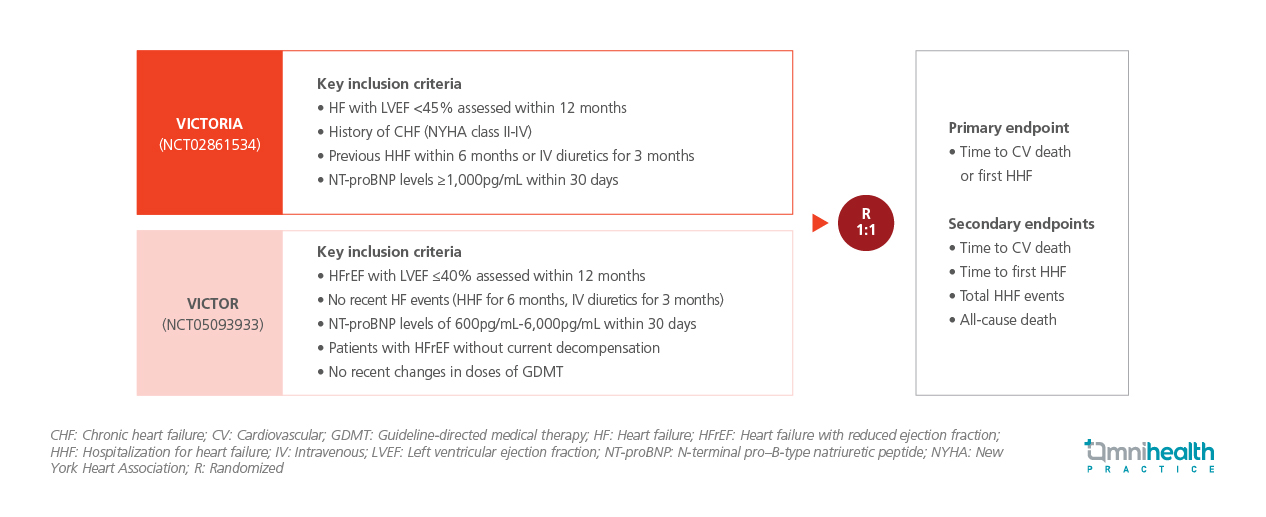
FINDINGS
| Primary endpoint : |
|
|
|
| Secondary endpoints : |
|
|
|
|
|

