CASE REVIEW
A local case sharing: earlier evolocumab for better long-term outcomes in patients with established ASCVD
Atherosclerotic cardiovascular disease (ASCVD) continuesto be a leading cause of morbidity and mortality worldwide,with over 17.9 million people dying from cardiovascular (CV)diseases annually.1,2 Given the fact that the recurrent riskis high among patients with established ASCVD, secondaryprevention with a potent low-density lipoprotein cholesterol(LDL-C) lowering regimen beyond statin alone is warrantedfor improving the overall CV outcomes.3,4 In recent years,evolocumab, a monoclonal antibody that inhibits proproteinconvertase subtilisin-kexin type 9 (PCSK9), has emergedas a promising therapeutic option with proven efficacy inlowering the LDL-C level and improving CV outcomesamong patients with established ASCVD, in combination withmaximally-tolerated statin.4 In an interview with Omnihealth Practice, Dr. Kwok, On-Hing Vincent shared a clinical case todemonstrate the long-term effectiveness of evolocumabin the real-world setting and discussed the latest resultsof the FOURIER open-label extension (OLE) study.
Background
The risk of the recurrent event among patients with established ASCVD [e.g., post-myocardial infarction (MI)] is high.5 A large real-world study (n=108,315) found that 24.7% of post-MI patients would experience another CV event, including stroke, recurrent MI, and death, within the first 365 days after index MI.5 For post-MI patients who did not experience another CV event during the first year, the risk remained elevated, with 1 in 5 experiencing another CV event during the subsequent years.5
LDL-C is a well-established and modifiable risk factor for ASCVD.4 For post-MI patients or those with established ASCVD, achieving the targeted LDL-C level goal is crucial for reducing the risk of recurrent risk and slowing the disease progression of atherosclerosis. A number of studies, including prospective and observational trials, have demonstrated a direct and significant relationship among the absolute LDL-C level reduction, the ASCVD event risk, and atherosclerosis progression.6 In particular, clinical trials have shown that patients who have achieved LDL-C levels of <1.4mmol/L experienced significantly lower event rates vs. those at levels of ≥1.4mmol/L.6
Based on the compelling evidence, the 2021 European Society of Cardiology (ESC) guidelines recommend that patients with established ASCVD should achieve an ultimate LDL-C goal of <1.4mmol/L and ≥50% reduction from baseline.7 More recently, the latest 2022 American College of Cardiology (ACC) expert consensus has aligned with the LDL-C recommendations of the 2021 ESC guidelines for ASCVD patients at very high risk on statin therapy for secondary prevention.6 However, Dr. Kwok stressed that the LDL-C level of 1.4mmol/L should not be a hard target. “Instead, we should aim for an even lower LDL-C level to minimize the recurrent CV risk as long as the patients can tolerate the treatment”, Dr. Kwok asserted.
In general, patients with established ASCVD are initially managed with statin monotherapy for secondary prevention, if tolerated.6 In case of patients who fail to achieve the recommended LDL-C target, non-statin therapies such as the PCSK9 inhibitors with profound LDL-C lowering effects should be considered.6 There are several non-statin treatment options available in this setting. However, guidelines recommend that preferences should be given to therapies with demonstrated CV outcome benefits.6 In the 2022 ACC expert consensus, PCSK9 monoclonal antibodies (mAbs) such as evolocumab, with or without ezetimibe, have been placed as the second-line options for treatment of very high-risk patients.6
To demonstrate the long-term effectiveness of evolocumab in local practice, Dr. Kwok shared a clinical case of a male patient who had undergone triple-vessel stenting and was treated with evolocumab as early as 2017 when the drug was first launched in Hong Kong. Since then, his condition has stabilized with no reported CV event.
Case sharing
A 63-year-old chronic male smoker suffered from acute inferior ST-elevation myocardial infarction (STEMI) in February 2005 (figure 1). Upon initial examination, the patient was found to have right coronary artery (RCA) total occlusion and subsequently received primary angioplasty with good results. Further findings identified left coronary artery (LCA) occlusion of 80%, which was also stented, and left anterior descending coronary artery (LAD) occlusion of 40%-50%. The baseline cholesterol levels of this patient were high with an LDL-C level of 3.7mmol/L. He was also obese and diabetic with a random blood sugar of 9.7mmol/L.

In 2005, the patient was treated with dual antiplatelet therapy (DAPT), high-dose statin (i.e., atorvastatin 40mg/day + ezetimibe 10mg/day), beta-blockers, and angiotensin-converting enzyme inhibitors. The patient was followed up in 2006, with the LDL-C level dropped to 2.4mmol/L. Yet, it was still well above the guidelinerecommended target level.
In 2009, the optimization of oral medications and lifestyle changes eventually lowered the patient’s LDL-C level to 1.7mmol/L and fulfilled the guideline recommendations. Nevertheless, in 2010, the patient complained of chest pain and was hospitalized. The occlusion in the LAD deteriorated, and percutaneous coronary intervention (PCI) was performed again (i.e., triple-vessel disease).
In fact, the incident signaled the need for a more aggressive LDL-C level target in this very high-risk ASCVD patient. Since the lipid-lowering treatment options were limited in 2010, it was difficult to control the LDL-C level to below 1.8mmol/L, let alone a stricter target. Reluctantly, throughout the 10-year conventional treatment, the patient’s LDL-C levels were only maintained between 1.7-2.3mmol/L.
Evolocumab was launched in 2016/2017, providing a promising treatment option for very high-risk patients with a prominent LDL-C-lowering effect. In July 2017, the patient was initiated with evolocumab 140mg subcutaneously every 2 weeks. In just 6 months of evolocumab, his LDL-C level was significantly reduced to 0.46mmol/L, which was sustained throughout the 5 years of evolocumab therapy. During the treatment course, the patient tolerated evolocumab well (except for mild injection-site reaction), and no serious adverse event (SAE) or CV event was reported. Currently, the patient’s condition is stable, taking evolocumab 140mg every 2 weeks on top of a low dose of rosuvastatin 5mg.
Discussion
The CV outcome benefits of evolocumab were demonstrated in the FOURIER study, a randomized, double-blind, placebocontrolled trial enrolling 27,564 patients with established ASCVD and LDL-C ≥1.8mmol/L.4 All patients were on background statin therapy.4 Participants were randomized 1:1 to receive evolocumab 140mg every 2 weeks/420mg every month or matching subcutaneous placebo.4 The primary study endpoint was the composite of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization.4
With a median follow-up of 2.2 years, evolocumab significantly reduced the risk of primary CV outcome by 15% vs. placebo (HR=0.85; 95% CI: 0.79-0.92; p<0.001) and was well tolerated with placebo-like safety profile (except for a higher rate of injection-site reaction with evolocumab).4 Of note, no difference in CV death was observed (HR=1.05; 95% CI: 0.88-1.25; p=0.62) in the initial results of FOURIER.4
To evaluate the long-term safety and efficacy of evolocumab in established ASCVD, the FOURIER-OLE study was performed with maximum exposure time of 8.4 years and follow up time of 8.7 years.8 A total of 6,635 patients were included in FOURIER-OLE, with 3,355 patients from the evolocumab group and 3,280 from the placebo group in the parent study.8 All enrolled patients were administered evolocumab.8 The primary study endpoint was the subjective incidence of treatment-emergent adverse events (TEAEs), and the primary CV composite outcome was CV death, MI, stroke, hospitalization for unstable angina (UA), or coronary revascularization.8 Major adverse cardiovascular events (MACEs) such as CV death were prespecified exploratory endpoints.8
Upon switching to evolocumab, the LDL-C levels of patients originally treated with placebo dropped rapidly and significantly from a median level of 2.30mmol/L to 0.78mmol/L (i.e., from 89 to 30mg/dL) which was sustained throughout the study (figure 2).8

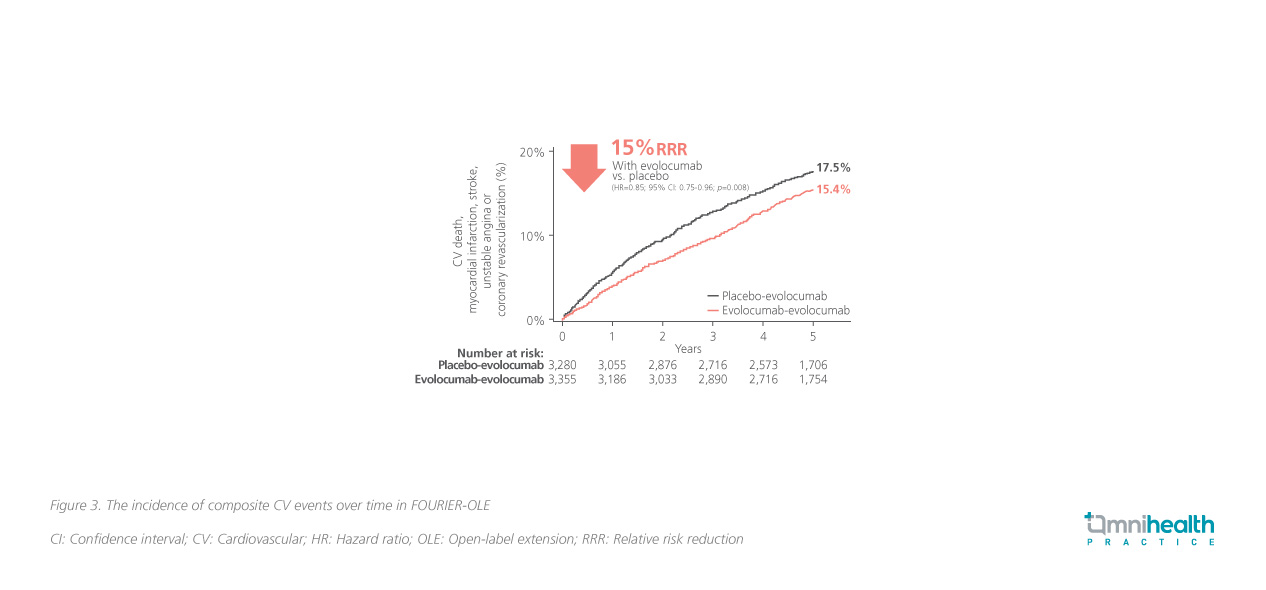
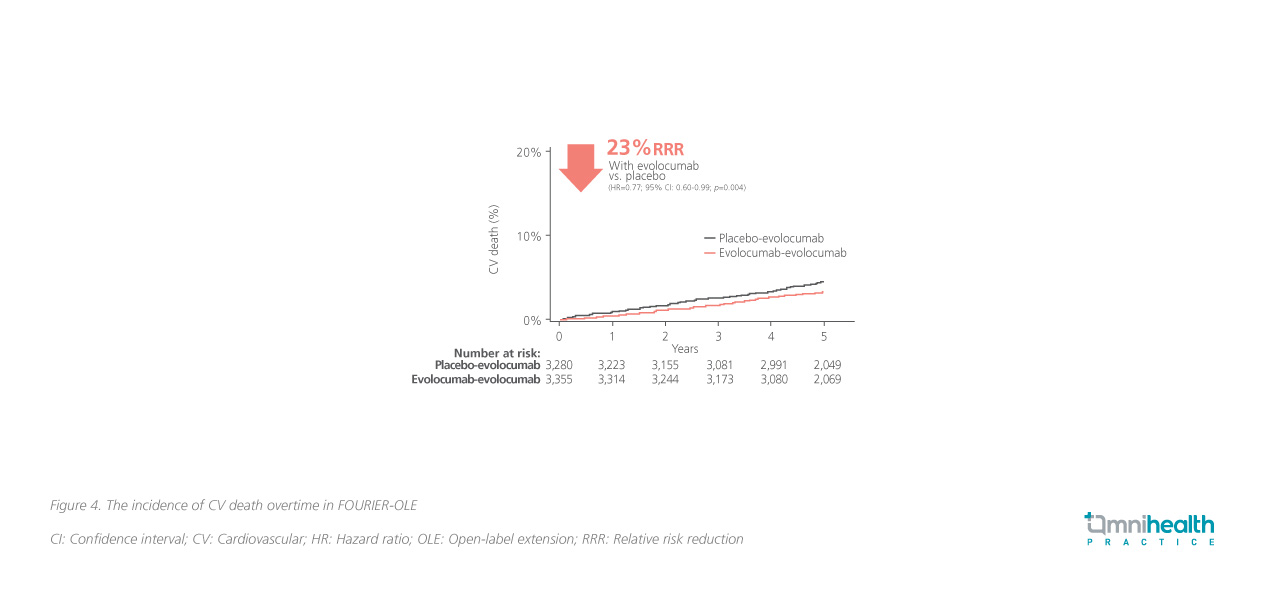
In FOURIER together with FOURIER-OLE, the maximum exposure to evolocumab was 8.4 years.8 Results showed that the incidence of SAEs, muscle-related events, new-onset diabetes, hemorrhagic stroke, and neurocognitive events with long-term evolocumab was similar to those for placebotreated patients in FOURIER-OLE and did not increase over time.8 With an additional 5 years of follow-up, evolocumab demonstrated an additional CV outcome improvement in the primary CV composite endpoint for patients who initiated evolocumab earlier in the parent study, when compared with those who started evolocumab later in FOURIEROLE (HR=0.85; 95% CI: 0.75-0.96; p=0.008) (figure 3).8 In addition, the earlier initiation of evolocumab showed a 23% lower risk of CV death vs. patients who had received placebo, which was not shown in the results of the parent study due to the short follow-up time (HR=0.77; 95% CI: 0.60-0.99; p=0.04) (figure 4).8
Conclusion
Dr. Kwok stressed that LDL-C is a well-established and modifiable risk factor for CV diseases, and that LDL-C reduction remains the mainstay for secondary prevention in patients with established ASCVD. In the past, treatment options were limited, making it difficult to lower the LDL-C levels effectively and optimize patients’ CV outcomes. With the availability of novel agents such as evolocumab, the achievement of aggressive LDL-C targets and effective prevention of recurrent CV events have become more feasible. All in all, as demonstrated in FOURIER-OLE, an earlier initiation of evolocumab in high-risk ASCVD patients is associated with clinically meaningful CV benefits, and therefore should be adopted as soon as possible in real-world practice.

