CONFERENCE UPDATE: ESC 2023
Long-term efficacy and tolerability of inclisiran sustained in ORION-8, the 3-year OLE study, among patients with high CV risk
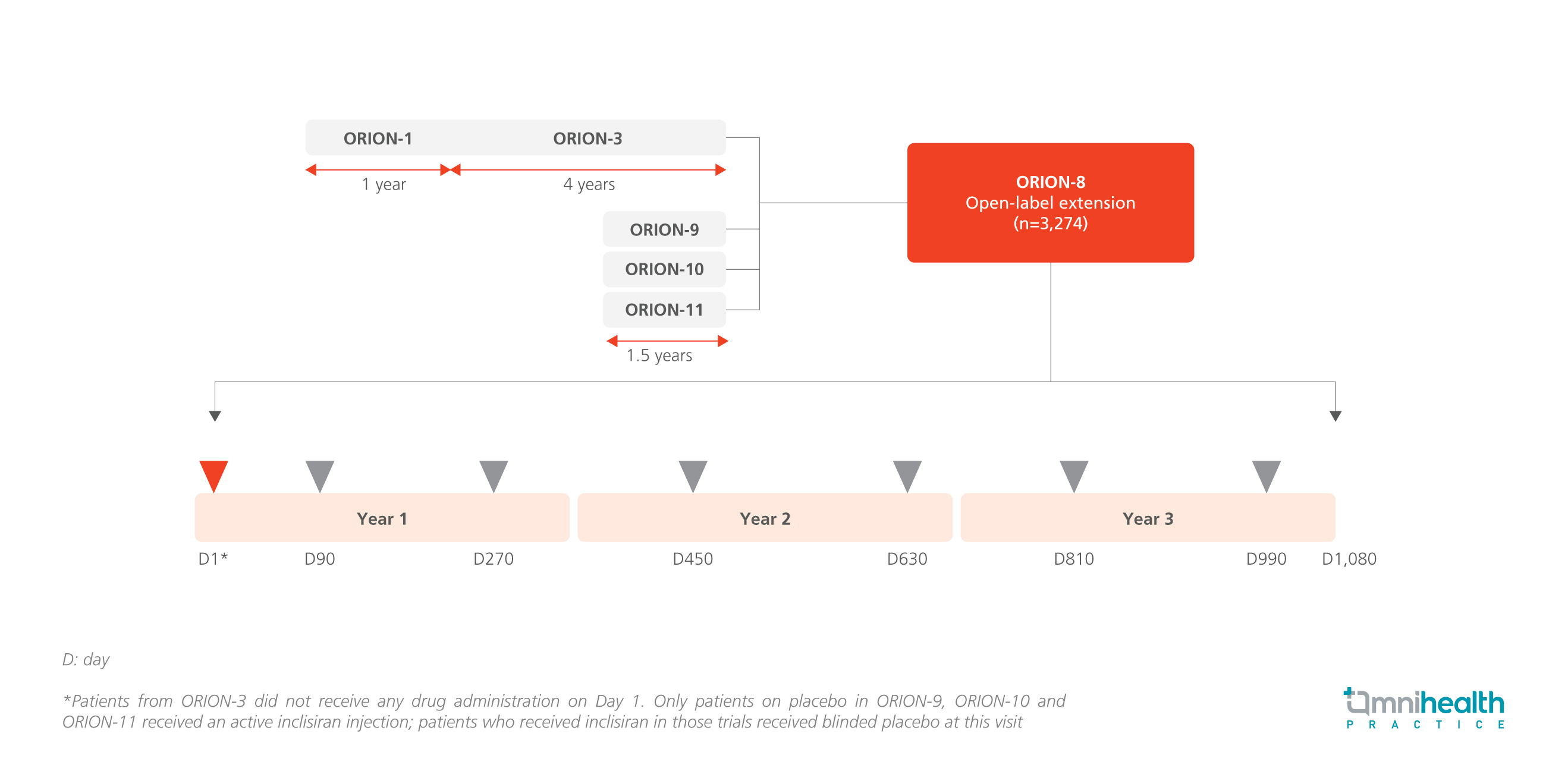
STUDY DESIGN
Inclisiran is a small interfering ribonucleic acid (siRNA) therapeutic, which offers a 52% reduction in low-density lipoprotein cholesterol (LDL-C) after only 18 months of treatment among patients with high cardiovascular (CV) risks.1 Previous phase 3 studies such as ORION-9, ORION-10, and ORION-11 had already established the efficacy and safety of the drug but data on its long-term use was only investigated in the phase 2 open-label extension (OLE) study ORION-3.1 Therefore, much of inclisiran’s extended effect remains unexplored in the clinical setting. At the ESC 2023 Congress, Dr. Scott R. Wright presented the results of ORION-8, a 3-year OLE to a pooled population of multiple phase 2 and phase 3 trials.1
The ORION-8 study assessed the long-term efficacy and tolerability of inclisiran in patients with atherosclerotic cardiovascular disease (ASCVD) or ASCVD risk equivalent as defined by being in the high-risk primary prevention category.1 The study incorporated patients from the ORION-3, ORION-9, ORION-10, and ORION-11 trials, enrolling a total of 3,274 patients with 82.7% of them having established ASCVD and the rest being ASCVD risk equivalent.¹ During the 3-year study period, patients received inclisiran injections twice a year, resulting in a cumulative inclisiran exposure of 12,109.3 patient-years.1 The primary endpoints for this OLE included the proportion of patients achieving pre-specified LDL-C goals (ASCVD: <1.8mmol/L; ASCVD risk equivalent: <2.6mmol/L) at the end of study and safety.¹ The percentage change in LDL-C from baseline to the end of study served as the secondary endpoint.1
FINDINGS
| Primary endpoint: |
|
|
|
|
| Secondary endpoints: |
|
|
|
| Safety: |
|
|
|
|
"ORION-8 provides additional evidence to support the long-term efficacy and tolerability of inclisiran in patients with high CV risk and elevated LDL-C."
Dr. Scott R. Wright
Mayo Clinic,
Rochester, Minnesota, United States

