CASE REVIEW
A local case sharing: Key strategies to identify statin intolerance followed by aggressive lipid-lowering treatment
High levels of low-density lipoprotein cholesterol (LDL-C) is a potent risk factor for atherosclerotic cardiovascular disease that contributes to approximately 45% of all heart attacks.1 While statins remain the cornerstone therapy for dyslipidemia and are generally well-tolerated, 25-30% of patients would show some forms of statin intolerance resulting in treatment discontinuation.2 As lipid-lowering treatment discontinuation may increase the risk of cardiovascular events, it is clinically important to make a correct diagnosis of statin intolerance to prevent the worsening of cardiovascular outcomes.3 In a recent interview with Omnihealth Practice, Dr. Yuan Gao, Specialist in Neurology, Queen Mary Hospital, shared the critical components of assessing statin intolerance and the need to switch to a non-statin alternative such as proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) regimen if aggressive lipid-lowering treatment is required.
Background
In 2017, 4.32 million global deaths and 94.9 million disability-adjusted life-years were attributed to high LDL-C.1 In Hong Kong, 35% of non-institutionalized persons aged 15-84 years have borderline high or above level of LDL-C and are at increased risk of cardiovascular events.1 When considering cardiovascular pharmacotherapy, statins are the mainstay for dyslipidemia and have demonstrated a 12% reduction in all-cause mortality per mmol/L reduction in LDL-C over a 5-year treatment period.3
However, not all patients can tolerate statins with 40-75% discontinuing statins within 1-2 years, commonly due to statin-associated muscle symptoms (SAMS), statin-induced dyspepsia, nausea, alopecia, and erectile dysfunction.3,4 While stopping statins may relief SAMS and other adverse events, treatment discontinuation for 4-6 weeks may cause atheroma plaque instability and increase the risk of cardiovascular events.3 Moreover, patients with acute myocardial infarction may experience rebound inflammation after statin withdrawal.5 On the other hand, lowering blood LDL-C remains an essential component to reduce the morbidity and mortality associated with atherosclerotic vascular disease, and a hasty clinical diagnosis of statin intolerance may hinder patients receiving optimal treatment in the future.6 Therefore, it is clinically important to identify patients who are truly statin intolerant so that the appropriate lipid-lowering treatment alternatives can be used to prevent unnecessary discontinuation of therapy and avoid the subsequent increase in cardiovascular risks.4
Identifying patients in need of non-statin aggressive lipid-lowering therapy
In practice, around 10-25% of patients would experience statin-related myocardial toxicity and require careful assessment of statin intolerance. When myalgia and a marked increase in creatinine kinase (CK) level are observed, statins should be withheld and the patient should be rechallenged by a different statin after SAMS subside. If myalgia and an elevated CK level are observed again, the patient is considered statin intolerant and should immediately switch to a non-statin lipid-lowering regimen to avoid treatment discontinuation. However, since SAMS are not necessarily accompanied by marked CK elevation, routine CK monitoring is not recommended by the European Atherosclerosis Society (EAS) due to its rarity during statin therapy.7
Although statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) inhibitors and CYP-related enzymes can affect the bioavailability and systemic clearance of statins, clinical experience showed that both the HMGCR and CYP-related genes are not strongly associated with statin-induced immune-mediated myopathy.8 While some studies suggested that polymorphisms in HMGCR can result in significantly diminished response to statins, a strong correlation in practice has yet to be established.9 Similarly, even though the SEARCH trial identified some common variants in SLCO1B1 to be linked with an increased risk of statin-induced myopathy, recent meta-analysis concluded that there was no association between the polymorphism of SLCO1B1 and myopathy.10,11 Due to this lack of clinical support, genetic testing for statin intolerance is rarely conducted in practice and not used for clinical diagnosis in Hong Kong.
On the other hand, patients who are exposed to statins can develop the anti-HMGCR antibody which is a marker for immune-mediated necrotizing myopathy (IMNM) and is a clear indicator for statin intolerance.12 From experience, the development of anti-HMGCR antibody can quickly turn a walking individual to wheelchair- or bed-bound within 2 months. As such, if the anti-HMGCR antibody is detected by routine blood tests, statins should be stopped immediately and the patient should not receive any statins in the future. Instead, these patients should be given immunosuppressants to quickly suppress the immune-mediated process associated with myofiber atrophy. If aggressive lipid-lowering treatment is indicated, the non-statin alternative PCSK9i will be recommended regardless of the patient’s age once statin intolerance is confirmed.
Case Report
In 2017, a non-smoking and non-drinking elderly lady who had hypertension and hyperlipidemia was prescribed atorvastatin 20mg once per day as the lipid-lowering treatment. After 2 months of therapy, she visited the Tian Tan Buddha at Tung Chung and found that she could not climb any stairs due to muscle weakness.
Initially, she thought the muscle weakness was related to age and paid little attention to the matter. However, her symptoms did not improve over time and she eventually visited the clinic. A blood test revealed that her CK level was elevated to around 7,000U/L. Although atorvastatin was immediately suspended, her symptoms continued to worsen over the next 2 months and she became wheelchair-bound. Shortly after, she was admitted to the hospital.
At admission, she was suspected of myocardial infarction based on the symptoms of hypertension, hyperlipidemia, chest discomfort, high CK level, and high troponin-T level that was found in another blood test. She was given enoxaparin and underwent percutaneous coronary intervention to resolve her potential heart failure. Unfortunately, her symptoms did not improve and a review of her medical history highlighted that she became wheelchair-bound within a very short period of time. She was then considered to have suspected myositis and was referred to a neurologist specialized in neuromuscular disorders.
Based on her symptoms, she was suspected of statin-associated IMNM. MRI muscle imaging and targeted muscle biopsy confirmed diagnosis of IMNM. Her blood samples were sent overseas to the Mayo Clinic in the United States to confirm the presence of anti-signal recognition particle (SRP) and anti-HMGCR antibodies. Returned results indicated an elevated anti-HMGCR antibody level of >200U/mL while being negative for anti-SRP antibody. She was then diagnosed of statin-induced, anti-HMGCR autoantibody-associated, IMNM and was given aggressive and combination immunotherapies including monthly 5-day intravenous immunoglobulin (IVIg) for 3 months, prednisolone and mycophenolate mofetil (MMF). Impressively, she quickly regained power and was able to walk again under assistance after the second monthly IVIg. She was successfully discharged from the hospital walking unaided after completing the third monthly IVIg.
After discharge, she was able to spend the Chinese New Year with her family at home but was readmitted afterward due to cardioembolic stroke with newly detected atrial fibrillation. She was given tissue plasminogen activator (tPA) and then discharged again shortly after with oral anticoagulant.
Given her recent stroke and persisting hyperlipidemia, she was given the PCSK9i evolocumab to aggressively lower her lipid level and prevent future cardiovascular events. After receiving PCSK9i, she did not experience any function impairing side effects and continued to enjoy a good quality of life. She maintained her daily activities with a LDL-C successfully lowered to 1.1-1.2mmol/L.
Discussion
From a neurologist’s perspective, not many clinicians are aware of SAMS or IMNM and most statin intolerant patients are referred to cardiologists due to similar symptoms to heart failure. In fact, some patients are even referred to rheumatologists due to inflammation or hepatologists due to elevated CK. In this patient case, the elderly lady was referred to a neurologist only after her SAMS had manifested for more than half a year. While her IMNM was rapidly resolved with immunosuppressants and her hyperlipidemia was controlled with evolocumab, a prolonged period of IMNM can expend muscle mass and make recovery difficult. Moreover, while immunosuppressants and steroids can reduce SAMS, IMNM would often relapse if appropriate treatments were not given. As such, it is clinically important to raise awareness of SAMS and recognize the signs and symptoms of statin intolerance.
While PCSK9i is a potent non-statin lipid-lowering treatment and is in fact the only aggressive alternative for statin-intolerant patients, clinicians should confirm the reasons behind statin prescription in the first place before considering a treatment switch. For patients who are initially given statins solely based on a hyperlipidemia assessment from general practice, they may not have the indication for aggressive treatment and can instead receive ezetimibe or lifestyle modifications. However, for statin-intolerant patients who have multiple underlying risk factors including diabetes, prior stroke and co-existing diseases which warrant aggressive lipid-lowering treatment, the more potent PCSK9i should be considered.
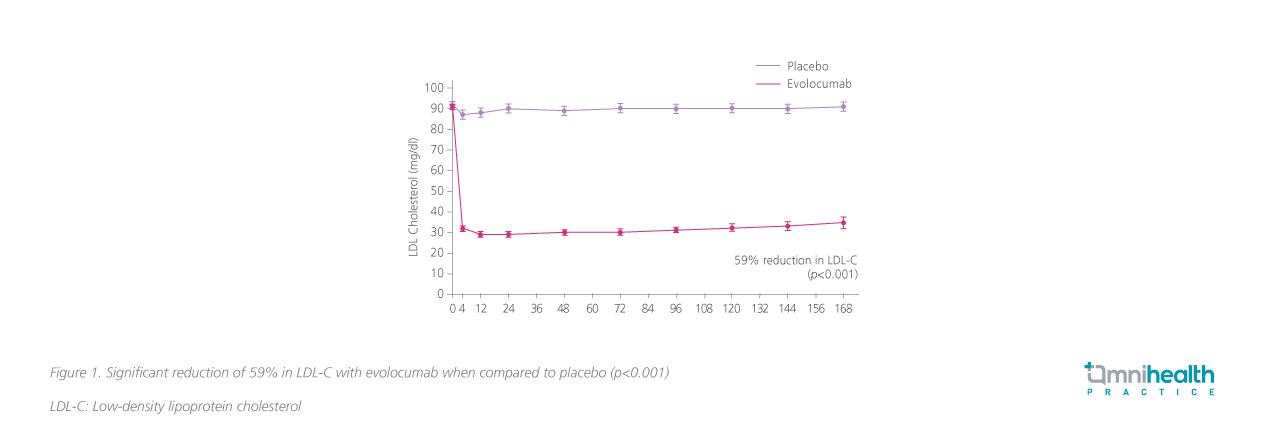
Indeed, the current management for patients with SAMS may include very low or intermittent administration of statins or ezetimibe.6 However, these treatments seldom achieve >50% reduction in blood LDL-C and may not be suitable for those requiring aggressive lipid-lowering therapy.6 On the other hand, the randomized, double-blind, placebo-controlled FOURIER study demonstrated that the PCSK9i evolocumab reduced LDL-C from 2.4 to 0.78mmol/L in 48 weeks of treatment, representing a significant 59% reduction in LDL-C when compared to placebo (p<0.001) that was maintained over time (Figure 1).13 Compared to placebo, evolocumab also significantly reduced the composite risk of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina or coronary revascularization (11.3% vs. 9.8%; HR=0.85; 95% CI: 0.79-0.92; p<0.001) as well as the composite risk of cardiovascular death, myocardial infarction or stroke (7.4% vs. 5.9%; HR=0.80; 95% CI: 0.73-0.88; p<0.001).13 In terms of safety, the 5-year follow-up on the OSLER-1 study showed that evolocumab had a similar yearly rate of serious adverse events when compared to standard of care during year 1 (6.9-7.9% vs. 6.8%), highlighting the long-term safety of evolocumab as a non-statin lipid-lowering treatment.14
The randomized, double-blind LAPLACE-2 study also showed that the addition of evolocumab to those who continued to receive statin every 2 weeks or monthly further reduced LDL-C by 66%-75% and 63%-75%, respectively, by 12 weeks of treatment that was significantly better than both the ezetimibe or placebo (p<0.001).15 Notably, evolocumab showed added benefit among patients who received moderate- and even high-intensity statins with the lipid-lowering benefit extended to non-high-density lipoprotein cholesterol, apolipoprotein B and lipoprotein.15 Similar to the FOURIER study, evolocumab showed comparable rates of adverse events to ezetimibe and placebo in LAPLACE-2, indicating that evolocumab is a safe and effective approach to lower LDL-C when statins are not optimal.15
Given the importance of a correct statin-intolerant diagnosis, patients who are exposed to statin with marked CK elevation and signs of myalgia should be immediately referred to a neurologist. If an increase in CK level of more than 3 times above the normal range within 2 months along with clear muscle weakness and an alanine aminotransferase (ALT): aspartate aminotransferase (AST) ratio of >1 are observed, the patient can be confirmed as statin intolerant and should promptly switch to a non-statin option even before anti-HMGCR antibodies are confirmed by blood tests. “Significantly elevated CK level, highly impaired muscle function and an ALT:AST ratio of >1 are strong predictors for statin intolerance, and these patients should switch to PCSK9i if aggressive lipid-lowering treatment is required,” concluded Dr. Gao.
Conclusion
Although statins are considered the mainstay of lipid-lowering treatment, patients who are statin-intolerant would require alternatives such as PSCK9i to aggressively manage hyperlipidemia and prevent the worsening of cardiovascular outcomes. To better diagnose statin intolerance, a consultation with neurologist specialized in neuromuscular disorders and an evaluation of the patient’s CK level, muscle function and ALT:AST ratio are important. Adopting the strategy of early, aggressive, and combination treatment with PCSK9i, statin-intolerant patients can now easily achieve their treatment goal with a lower risk of IMNM.

