CONFERENCE UPDATE: EASD 2025
Baricitinib leads to significant scalp hair regrowth in adults and adolescents with severe AA: Results from the BRAVE-AA trials
STUDY DESIGN
Baricitinib, a selective Janus kinase (JAK) inhibitor approved for severe alopecia areata (AA) in adults, has been evaluated in adolescents aged 12-18 years.1 Across both adult and adolescent studies, baricitinib demonstrated clinically meaningful hair regrowth, with ≥80% scalp coverage in a substantial proportion of patients.1 However, the commonly used primary endpoint of Severity of Alopecia Tool (SALT) score of ≤20 may not fully reflect complete regrowth.1 As achieving full scalp hair regrowth remains a key treatment goal, the BRAVE-AA trials further assessed the complete response thresholds of SALT ≤10 and ≤5 among SALT ≤20 responders through week 52 in both adults and adolescents.1
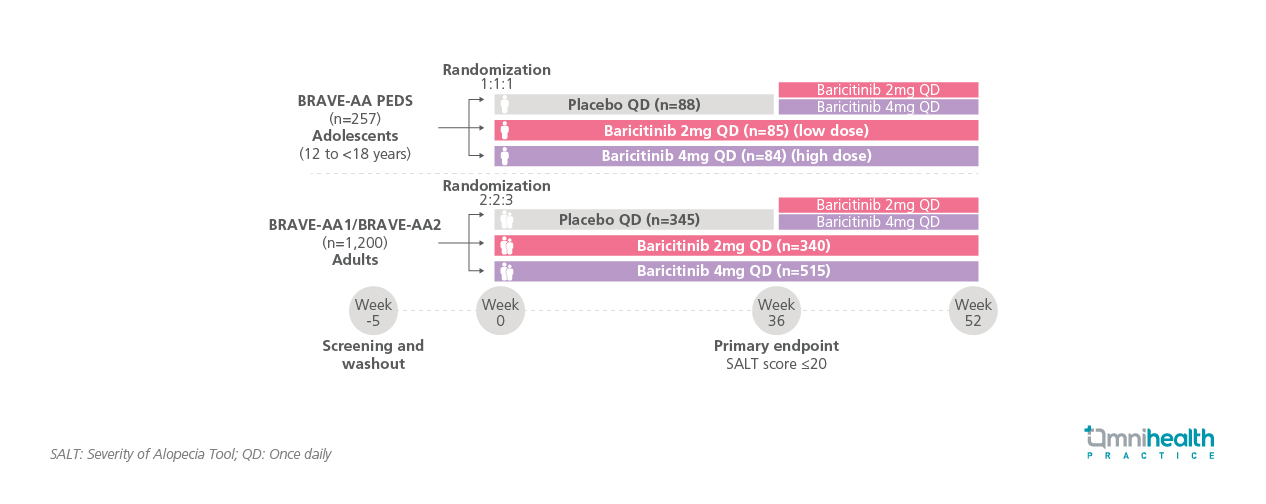
The BRAVE-AA program included two adult studies (BRAVE-AA1 and BRAVE-AA2) and one adolescent study (BRAVE-AA-PEDS).1 Common inclusion criteria required a SALT score ≥50 at screening and baseline, an AA episode lasting >6 months to <8 years, and no spontaneous improvement within 6 months.1 For BRAVE-AA-PEDS, eligible patients were aged 12 to <18 years, weighed ≥30kg, had a confirmed AA diagnosis for ≥1 year, and had failed more than one previous therapy.1 History of psychological counseling and evidence of psychological distress related to AA were also criteria for inclusion.1 For BRAVE-AA1 and BRAVE-AA2, adults aged ≥18 years (males <60, females <70) were eligible.1 Compared to the adult population, a greater proportion of adolescents had very severe baseline disease, atopic dermatitis, and asthma.1
Following a 5-week screening and washout period, 257 adolescents were randomized 1:1:1 to placebo, baricitinib 2mg, or 4mg daily, with placebo patients switched to active treatment at week 36.1 A total of 1,200 adults were randomized 2:2:3 to placebo, baricitinib 2mg, or 4mg daily, with placebo patients also switching to active treatment at week 36.1 All participants were followed for 52 weeks, and treatment response was assessed using SALT scores at prespecified timepoints.1 The primary endpoint was the proportion of responders with SALT ≤20 at week 36.1 The key outcomes assessed in this analysis were the proportion of responders with deeper responses (SALT ≤10 [near-complete scalp coverage] or ≤5 [complete scalp coverage]) with baricitinib 2mg or 4mg in week 4-52.1
FINDINGS
|
Primary endpoint: |
|
|
Other outcomes: |
|

