CASE REVIEW
Local case sharing: Benefits of early intensification with enzalutamide in mHSPC patients with high volume of disease
The advent of novel strategies has shifted the treatment paradigm of metastatic hormone-sensitive prostate cancer (mHSPC) from androgen deprivation therapy (ADT) monotherapy to combination therapies.1 Upfront addition of chemotherapy or androgen receptor (AR) inhibitors to ADT has demonstrated an improved overall survival (OS) and become a new standard of care (SoC) in this therapeutic area.2 The early treatment intensification is particularly important and beneficial to mHSPC patients with high volume of disease.3,4 In an interview with Omnihealth Practice, Dr. Lee, Siu-Hong discussed the unmet needs in mHSPC, emphasizing the importance of using combination therapy for the high-volume patients. He also stressed the significance of monitoring prostate-specific antigen (PSA) response and shared a clinical case of a 70-year-old male diagnosed with de novo mHSPC with high volume of disease who was managed with upfront ADT + enzalutamide, then achieving a rapid reduction in the serum PSA levels.
Background
Dismal prognosis with ADT alone
Prostate cancer (PC) is the third most common cancer among men in Hong Kong, with 2,315 new cases and 484 deaths in 2020.5 Nearly one-third of new PC patients were found to have metastatic diseases while 41.2% of patients had PSA levels of >20ng/mL, and 31.2% had Gleason score ≥8 at diagnosis.5
In general, patients with metastatic disease have a poorer prognosis and a 5-year survival rate of only 44.9%.6 Over the past 7 decades, ADT alone was the only SoC in mHSPC.1 Although up to 95% of patients responded to ADT, almost all patients experienced disease progression to fatal castration-resistant prostate cancer (CRPC) within 1-3 years.1,4 A clinical trial has revealed that mHSPC patients receiving ADT alone had only a median failure-free survival (FFS) of 11 months and a median OS of 42 months, indicating a huge unmet need among mHSPC patients.1
The limitations of chemotherapy in mHSPC
In CHAARTED study, the upfront addition of docetaxel to ADT in mHSPC patients with high-volume of diseases was shown to reduce the risk of death by 40% when compared to ADT alone with a median OS of 49.2 months (HR=0.60; 95 % CI: 0.45-0.81; p<0.001); yet, among those with low volume of disease, such benefits were statistically insignificant (HR=0.60; 95% CI: 0.32-1.13; p=0.11).3 Despite the demonstrated benefits in the high-disease volume setting, Dr. Lee cautioned that the safety and tolerability of chemotherapy must be considered, since most mHSPC patients are at an advanced age and are less likely to tolerate intensive treatment. Predictably, the adverse events (AEs) associated with docetaxel could impair the quality of life (QoL) of patients. An analysis found that there was a significant decrease in QoL, assessed by the Functional Assessment of Cancer Therapy-Prostate (FACT-P), between baseline and 3 months among patients treated with ADT + docetaxel (-2.7 points; p<0.001).7 Given the longer survival of mHSPC patients, Dr. Lee emphasized the need to maintain patients’ QoL throughout the treatment course and preferred treatment options with proven efficacy and better tolerability. Dr. Lee then shared a clinical case where he treated his mHSPC patient with a novel, second-generation AR inhibitor and avoided chemotherapy.
Case sharing
A 70-year-old male without past medical history had been experiencing minor lower urinary symptoms for many years. Since November 2022, the symptoms, especially difficulty in urination and low back pain, have worsened. The initial medical check-up found a very high PSA level of 230ng/mL. The results of biopsy confirmed a PC of Gleason scores of 4+5. Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) scan found multiple bone metastasis sites (>4) and multiple lymph node involvement in the pelvic area, spreading to the left supraclavicular fossa. He was diagnosed with de novo mHSPC with high disease volume.
The treatment priority for this patient was to halt disease progression as well as to improve his long-term survival. He was first treated with luteinizing hormone-releasing hormone (LHRH) antagonist, and the PSA level was declined to 50ng/mL after 2 months of treatment.
Enzalutamide was added after the first 2 doses of LHRH antagonist in January 2023. After only 1 month of enzalutamide, the PSA level was further decreased to <10ng/mL, and the urinary symptoms and back pain were resolved. During the first 2 months of enzalutamide treatment, the patient did not experience any AEs. Nonetheless, the long-term survival benefits and its impact on QoL with enzalutamide remain to be seen.
Concerning the elevated risks of serious AEs associated with long-term use of steroids including new-onset diabetes, bone fractures, cataract, etc.8 Dr. Lee reminded that since mHSPC patients have relatively longer survival, clinicians should consider the long-term safety of treatments when deciding the management plan for these patients.
Discussion
Enzalutamide is a second-generation oral AR inhibitor which, in combination with ADT, has previously demonstrated significant survival benefits in several randomized phase 2 & 3 trials and has been approved by local regulatory authorities for the management of non-metastatic or metastatic CRPC.9-14 Since then, enzalutamide has been widely utilized in clinical practice, showing high effectiveness and manageable safety profile among the real-world CRPC patients.15,16
In mHSPC, the safety and efficacy of enzalutamide + ADT were evaluated by ARCHES, a multinational, double-blind, randomized, placebo-controlled, phase 3 trial.4 A total of 1,150 men with mHSPC (high disease volume: >60%) were enrolled and randomized 1:1 to receive enzalutamide + ADT or ADT alone, stratified by disease volume and prior docetaxel chemotherapy.4 At the primary analysis (data cutoff date: October 14, 2018), results showed that enzalutamide + ADT significantly reduced the risk of radiographic progression or death in the overall population by 61% vs. ADT alone (HR=0.39; 95% CI: 0.30-0.50; p<0.001), including patients with high disease volume (HR=0.43; 95% CI: 0.33-0.57).4
Enzalutamide improves OS regardless of the disease volume
At the final prespecified OS analysis of ARCHES with a median follow-up of 44.6 months (data cutoff date: May 28, 2021), enzalutamide + ADT with a median treatment duration of 40.2 months significantly reduced the risk of death by 34% vs. ADT alone (HR=0.66; 95% CI: 0.53-0.81; p<0.001).17,18 Among patients with high volume of disease, the median OS was not reached with enzalutamide + ADT vs. 45.9 months with ADT alone (HR=0.66; 95% CI: 0.52-0.83) (figure 1).17,18
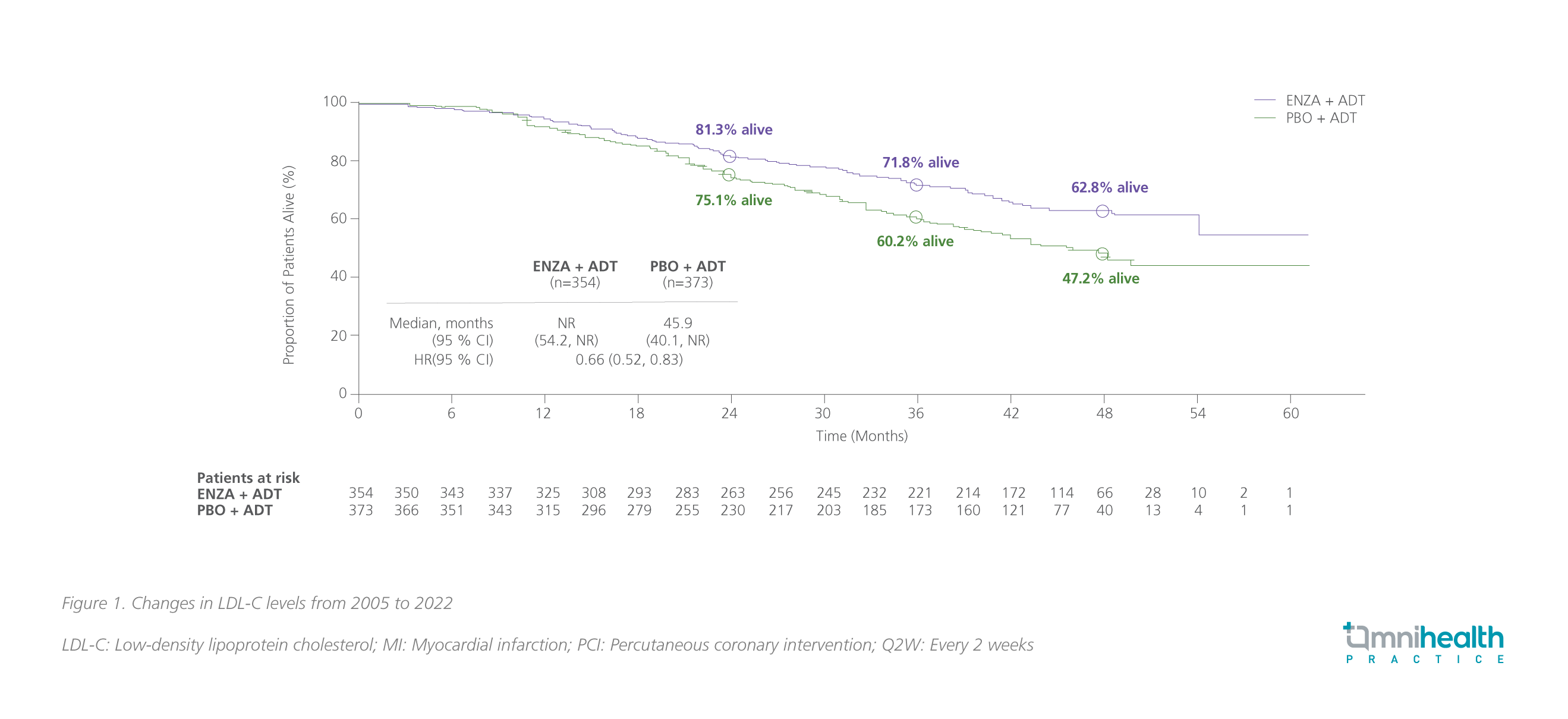
Of note, after unblinding of the ARCHES study, 180 patients (i.e., 31.3% of patients) in the placebo arm crossed over to the enzalutamide group during the open-label period.17 After adjusting the impact of crossover, the risk reduction in death with enzalutamide + ADT was 43% (95% CI: 0.45-0.70; p<0.001); the similar OS benefits with enzalutamide + ADT were observed across all subgroups.17 For patients with high-volume disease, the OS benefit was also more pronounced (HR=0.59; 95% CI: 0.46-0.74).18 Long-term use of enzalutamide + ADT showed a favorable safety profile, which was consistent with the previous reports and no new safety signal was identified in ARCHES.17
Enzalutamide significantly improves PSA-related outcomes in mHSPC
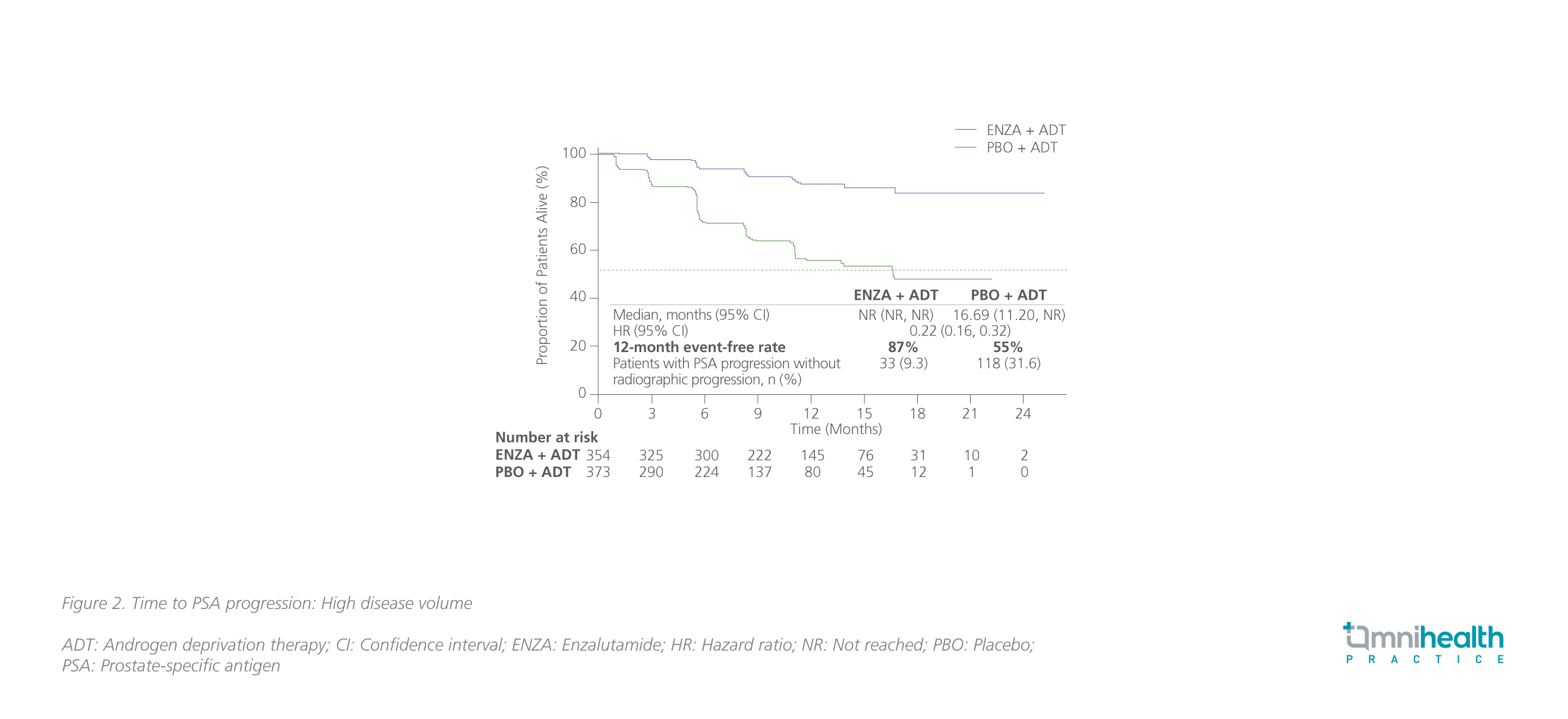
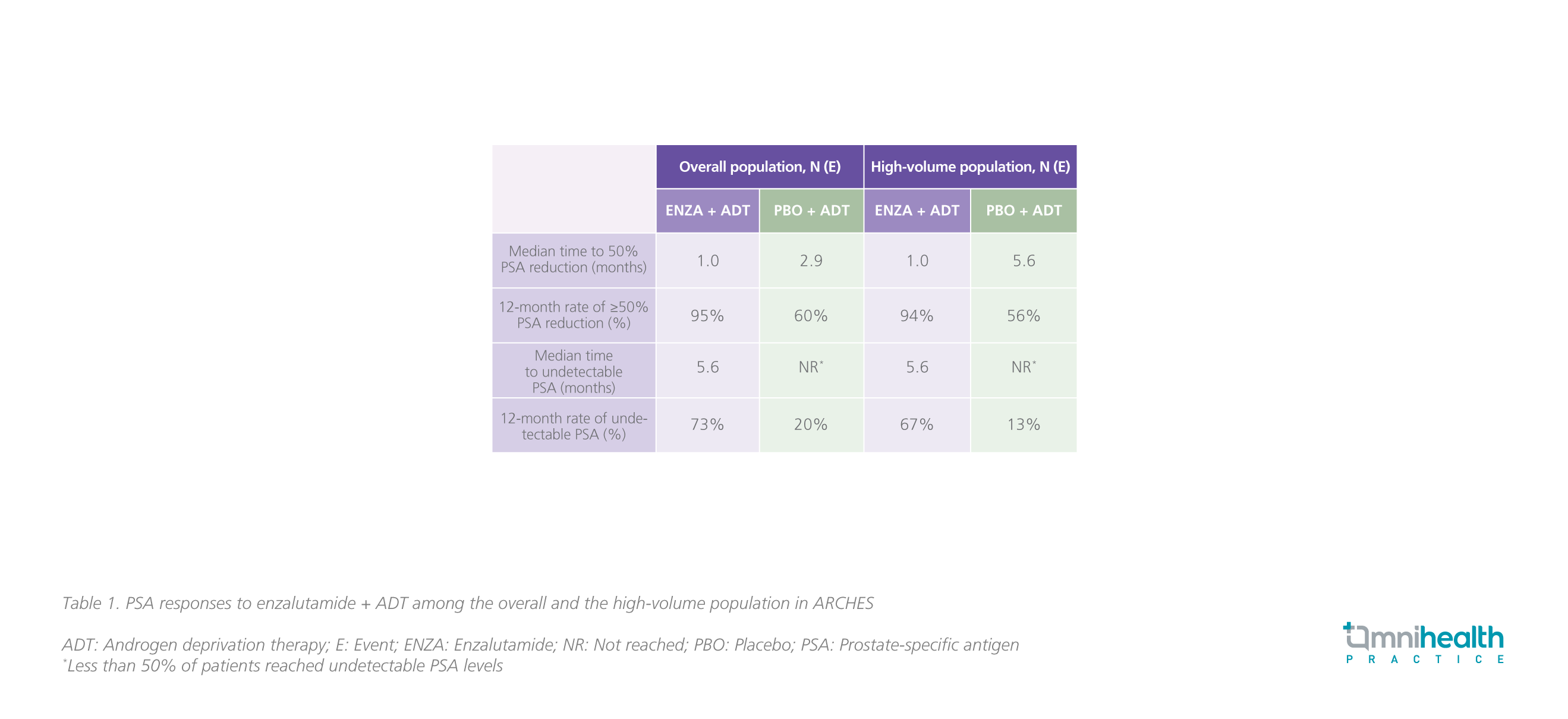
PSA has been recognized as a biomarker for diagnosis, prognosis, and monitoring of PC.20 In both CRPC and hormone-sensitive prostate cancer (HSPC), PSA progression, which was generally defined as an increase in PSA by ≥25% greater than nadir and an absolute increase of 2ng/mL despite the use of ADT, has been regarded as a well-accepted indicator of worsening disease and poor prognosis.20 In ARCHES, the addition of enzalutamide to ADT was shown to improve the PSA-related outcomes such as the time to PSA progression, the time to 50% PSA reduction, and the time to undetectable PSA (<0.2ng/mL) during the treatment course in the overall population vs. ADT alone.4,21 Results showed that enzalutamide + ADT significantly reduced the risk of PSA progression by 78% (HR=0.22; 95% CI: 0.16-0.32) among patients with high volume of disease, consistent with the overall population (HR=0.19; 95% CI: 0.13-0.26) (figure 2).21 For patients who have received prior docetaxel, an equally significant risk reduction of 78% in PSA progression was achieved with enzalutamide + ADT (HR=0.22; 95% CI: 0.11-0.45).21 The PSA responses to enzalutamide + ADT were summarized in table 1.21 “In my practice, the effect of enzalutamide in suppressing serum PSA levels is remarkable. Patients can normally have their PSA levels dropped by 50%-75% within a month,” Dr. Lee commented.
Clinical benefits of achieving undetectable PSA with enzalutamide
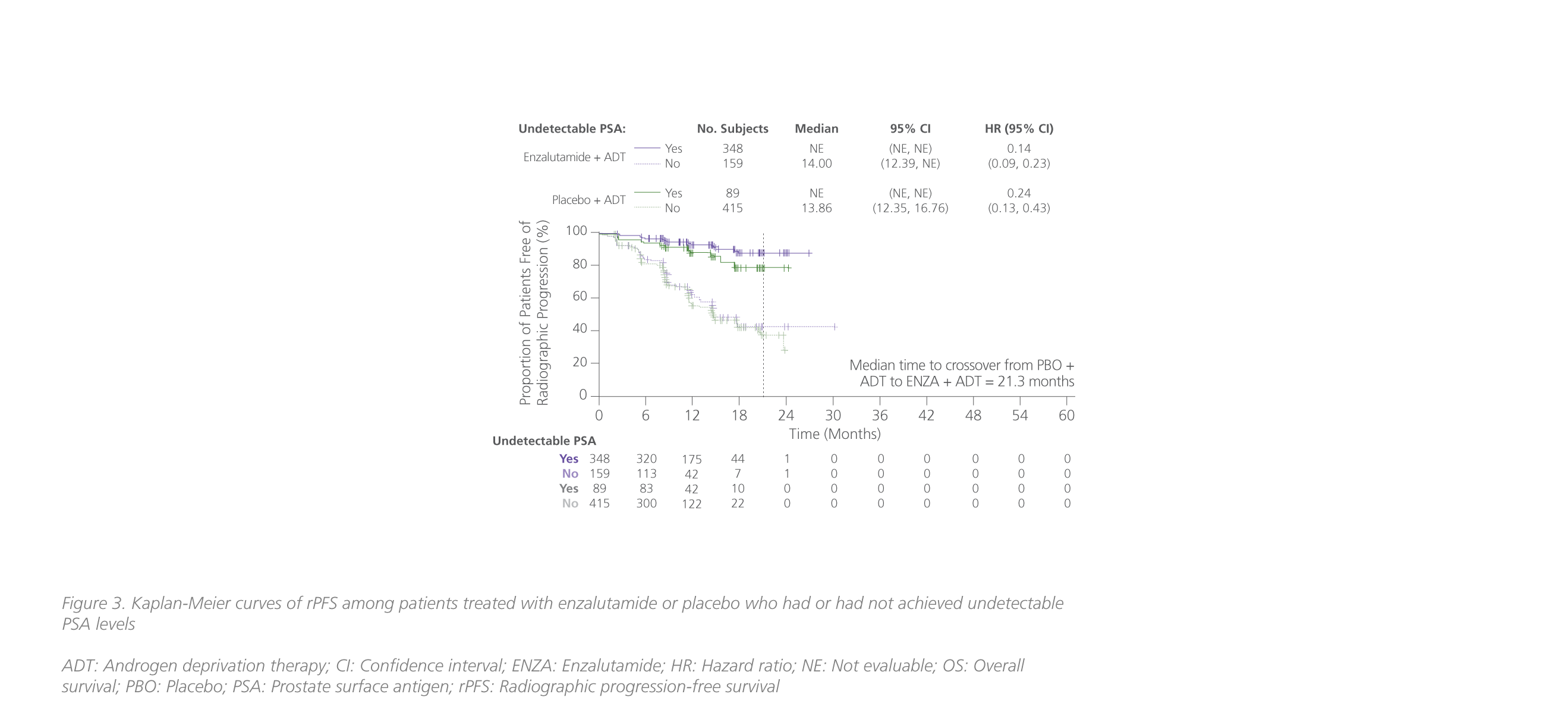
In a post-hoc analysis of ARCHES, the survival benefits associated with achieving undetectable PSA levels with enzalutamide + ADT in mHSPC were also assessed.22 In ARCHES, patients in the enzalutamide arm (68.1%) were approximately 4 times more likely to reach undetectable PSA levels than patients in the placebo arm (17.6%) (relative difference: 50.5%; 95% CI: 45.3-55.7; p<0.0001).22 Results showed that the risk of radiographic progression-free survival (rPFS) was significantly reduced by 86% among patients in the enzalutamide group who had achieved undetectable PSA levels vs. those who had not (HR=0.14; 95% CI: 0.09-0.23) (figure 3).22 The risk of death was also significantly lowered with enzalutamide-treated patients who had achieved undetectable PSA levels (HR=0.24; 95% CI: 0.17-0.34; p<0.0001) (figure 3).22
Apart from the profound survival benefits, attaining undetectable PSA with enzalutamide was also associated with a reduction in the risk of first deterioration in the FACT-P total scores (HR=0.78; 95% CI: 0.62-0.98).22 Notably, patients who achieved undetectable PSA levels on enzalutamide + ADT had higher FACT-P scores at baseline which were maintained over time, delaying deterioration in the overall QoL vs. those reaching detectable PSA levels after treatment.22 Such QoL benefits were not observed in patients treated with ADT alone.22
Enzalutamide delays chemotherapy and maintains a high QoL in mHSPC patients
Besides the demonstrated survival benefits, enzalutamide + ADT was also shown to significantly delay the time to use the first antineoplastic therapy including docetaxel in mHSPC patients (HR=0.38; 95% CI: 0.31-0.48).17 In a post-hoc analysis of ARCHES which evaluated patient-reported outcomes using the FACT-P and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Prostate 25 (QLQ-PR25), enzalutamide was shown to enable mHSPC patients to maintain a high functional health-related QoL (HRQoL) and a low symptom burden.19 When compared with ADT alone, enzalutamide + ADT significantly delayed the time to first deterioration in worse pain (HR=0.82; p=0.032); the median time to HRQoL (FACT total score) deterioration was also significantly delayed (HR=0.78; nominal p=0.020).19
Conclusion
Dr. Lee concluded that nowadays, ADT alone should no longer be regarded as the SoC for mHSPC patients. Instead, upfront addition of AR agents, such as enzalutamide, to ADT should be adopted, especially for patients with high-volume disease who have a higher disease burden and are more likely to have deterioration in QoL.
This is an independent editorial article, published and distributed through unrestricted educational support from the pharmaceutical community, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors
- Harada K, et al. Treatment strategies for metastatic castration-sensitive prostate cancer: from “all-comers” to “personalized” approach. OncoTargets Ther. 2021;14:2967-2974.
- Guideline-Prostate Cancer. European Association of Urology. https://uroweb.org/guidelines/prostate-cancer. Accessed March 22, 2023.
- Sweeney CJ, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
- Armstrong AJ, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Prostate cancer in 2020. Hong Kong Cancer Registry, Hospital Authority. https://www3.ha.org.hk/cancereg/pdf/factsheet/2020/prostate_2020.pdf. Accessed March 22, 2023.
- Hong Kong Cancer Registry. Report of stage-specific survival of prostate and thyroid cancers in Hong Kong. Hospital Authority, Hong Kong https://www3.ha.org.hk/cancereg/. Accessed March 28, 2023.
- Morgans AK, et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36(11):1088-1095.
- Rice JB, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216-2229.
- Sternberg CN, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197- 2206.
- Penson DF, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098-2106.
- Shore ND, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016; 17(2), 153-163. https://doi.org/10.1016/S1470-2045(15)00518-5
- Beer TM, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL Study. European urology. 2017; 71(2), 151–154. https://doi.org/10.1016/j.eururo.2016.07.032Affrim
- Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. Lancet Oncol. 2012; 367(13), 1187-1197.
- Xtandi® Hong Kong Prescribing Information. Astellas.
- Raju R, et al. Real-world data on outcomes in metastatic castrate-resistant prostate cancer patients treated with abiraterone or enzalutamide: a regional experience. Front Oncol. 2021;11:656146.
- Yokomizo A, et al. Real-world use of enzalutamide in men with nonmetastatic castration-resistant prostate cancer in Japan. Int J Clin Oncol. 2021;27:418-426.
- Armstrong AJ, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622.
- Armstrong AJ, et al. Overall Survival In Patients With Metastatic Hormone-Sensitive Prostate Cancer Treated With Enzalutamide Plus Androgen Deprivation Therapy by High or Low Disease Volume and Progression to Metastatic Hormone-Sensitive Prostate Cancer (M0 at Diagnosis) or De Novo Metastatic Hormone-Sensitive Prostate Cancer (M1 at Diagnosis): Post Hoc Analysis of The Phase 3 ARCHES Trial. Poster presented at the American Society of Clinical Oncology Genitourinary Cancer Symposium. February 17-19, 2022.
- Stenzl A, et al. Effect of enzalutamide plus androgen deprivation therapy on health-related quality of life in patients with metastatic hormone-sensitive prostate cancer: an analysis of the ARCHES randomised, placebo-controlled, phase 3 study. Euro Urol. 2020;78(4):603-614.
- Hussain M, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27(15):2450-2456.
- Shore ND, et al. Impact of baseline disease volume and prior docetaxel therapy on prostate-specific antigen-related outcomes in patients cancer treated with enzalutamide plus androgen deprivation therapy. Presented at the 115th American Urological Association Annual Meeting.
- Stenzl A, et al. Clinical Outcomes of Patients With Metastatic Hormone-Sensitive Prostate Cancer With Prostate-Specific Antigen Decline to Undetectable Levels on Enzalutamide: Post Hoc Analysis of ARCHES. Presented at European Association of Urology (EAU) 2022. July 1-4, 2022.

