CASE REVIEW
A local case sharing: Enzalutamide optimizes care for mHSPC patients with low-volume disease
In Hong Kong, prostate cancer is the third most common cancer in men, with 2,315 new cases diagnosed in 2020 according to the Hong Kong Cancer Registry.1 About one-third of these patients were classified as stage IV prostate cancer.1 Metastatic hormone-sensitive prostate cancer (mHSPC) is usually categorized as high- or low-volume disease, with the latter having a better prognosis.2 As such, healthcare professionals (HCPs) generally prefer androgen deprivation therapy (ADT) alone to manage the low-volume mHSPC patients. In recent years, early intensified regimens such as enzalutamide + ADT have demonstrated promising efficacy in delaying disease progression and improving survival outcomes in mHSPC patients, regardless of volume of disease.3-6 In a symposium organized by the Hong Kong Society of Uro-Oncology & Hong Kong Urological Association, Dr. Poon, Ming-Chun Darren discussed the need for optimizing the care for patients with mHSPC and low-volume disease. He also shared a clinical case showing the effectiveness of enzalutamide + ADT in clinical practice.
Background
Over the past several decades, ADT alone has been the standard of care (SoC) for mHSPC patients until disease progression to castrate-resistant prostate cancer (CRPC).3 Lately, several systemic therapies initially approved for the treatment of metastatic CRPC (mCRPC) have demonstrated a remarkable efficacy and balanced safety profile in men with mHSPC, establishing a new SoC for mHSPC.3
Enzalutamide, a second-generation androgen receptor (AR) targeted inhibitor, is among the novel systemic treatment options for managing mHSPC.4-7 In the ARCHES study, a multinational, double-blind, phase 3 trial, enzalutamide, in combination with ADT, significantly prolonged radiographic progression-free survival (rPFS), delayed prostate-specific antigen (PSA) progression and improved overall survival (OS) in men with mHSPC vs. placebo + ADT, including patients with low-volume disease.4-7 With a median treatment duration of 40.2 months, enzalutamide + ADT showed a well tolerable safety profile, which was consistent with the findings from the primary analysis of ARCHES.7 The rates of ≥grade 3 adverse events (AEs), serious AEs and AEs leading to treatment discontinuation were all similar in the enzalutamide and placebo groups.4 The rate of all-grade rash was only 3.8%, and no ≥grade 3 skin rash was reported, while a consistent safety profile with mCRPC patients was observed.7
Upon regulatory approval of this indication, enzalutamide has been used for the treatment of mHSPC patients in local practice. With the compelling evidence from various clinical trials, Dr. Poon stressed that mHSPC patients, including those with low-volume disease, should no longer be treated with ADT alone. In a recent symposium, he presented a clinical case of a male mHSPC patient with low-volume disease, demonstrating the effectiveness of enzalutamide in helping patients remain in long-term remission.
Case report
A 56-year-old, hypertensive man who was a hepatitis B carrier presented with lower urinary tract symptoms, such as increased urinary frequency and nocturia, in 2020. He did not have family history of prostate cancer. The initial biochemical findings of this patient showed an elevated PSA level of 28.5ng/mL. Magnetic resonance imaging (MRI) scan revealed bilobed Prostate Imaging-Reporting and Data System (PI-RAD) 5 lesions. MRI scan also found multiple pelvic lymph node (LN) involvements, which were of small size and were not regarded as pathogenic. Prostate biopsy was performed, showing adenocarcinoma with a Gleason score of 4+5. The prostate-specific membrane antigen positron emission tomography (PSMA-PET) scan was consistent with the MRI findings, showing bilobed PSMA uptake in the prostate lesions with extracapsular spread (ECS) and bilateral seminal vesicle (SV) invasion. In addition, the PSMA-PET scan found a solid bony metastasis at the thoracic level (i.e., T8 vertebral uptake) (figure 1). The findings were highly suggestive of bone metastasis and compatible with the computer tomography (CT) scan which identified a sclerotic lesion.
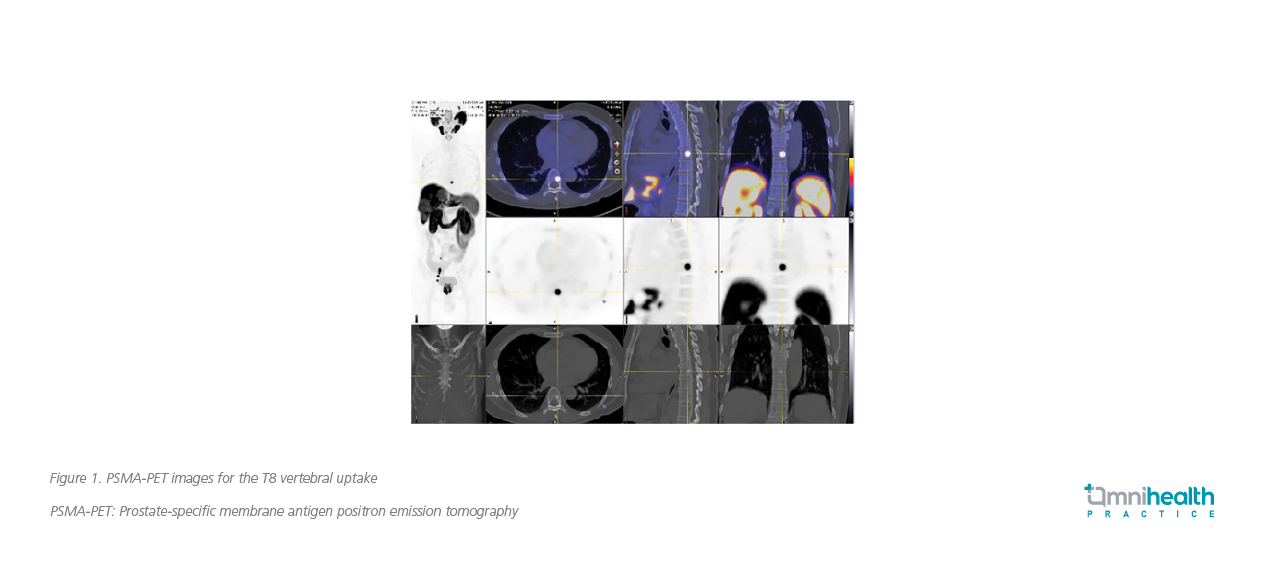
The overall impression was T3bN1M1 disease with a Gleason score of 4+5 and PSA of 28.5ng/mL. By definition, the patient was diagnosed with mHPSC as low-volume disease.
The patient started with ADT in August 2020. Concurrently, he also received stereotactic body radiation therapy (SBRT) to prostate/pelvic LN with magnetic resonance (MR) linear accelerator (Linac), which was completed in October 2020. The radiation doses were 40Gy to prostate, 33.5-36.25Gy to gross LNs, and 25Gy to pelvic lymphatics. Since he had solid bony metastasis at T8, SBRT was also given to T8 with the volumetric modulated arc therapy (VMAT) in November 2020, in a dose of 12Gy in 2 fractions.
Enzalutamide 160mg daily was initiated in November 2020. Since then, he achieved a very deep and sustained PSA response (figure 2). Throughout the 2-year enzalutamide treatment, he only experienced grade 1 fatigue and a slight elevation of blood pressure (BP). Notably, the diastolic BP increased to 85mmHg, but it was still within the normal range. His liver and renal functions remained normal. Amid receiving the treatments, the patient could work normally, and the impact on his daily life was minimal. In his latest follow-up, PSMA-PET was performed again, showing a complete radiographic remission with no PSMA uptake in both prostate and oligometastasis at T8.
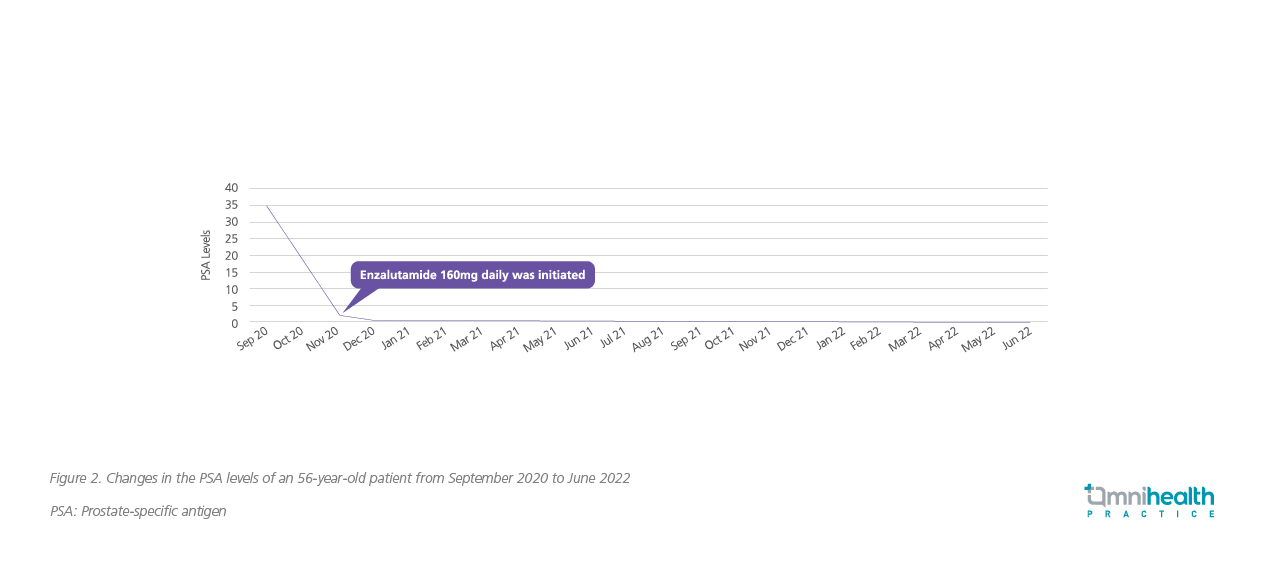
Overall, enzalutamide was effective in suppressing the PSA level and helping the mHSPC patient with low-volume disease to stay in remission and to have high quality of life. The medication was also well tolerated.
Discussion
In the primary analysis of ARCHES, enzalutamide + ADT significantly reduced the risk of rPFS by 61% vs. placebo + ADT, regardless of the disease volume (HR=0.39; 95% CI: 0.30-0.50, p<0.001).4 Among patients with low-volume disease, the risk reduction in rPFS was 75% (HR=0.25; 95% CI: 0.14-0.46), which was consistent with the overall population.4 Positive findings were also observed in the secondary endpoints, including time to PSA progression (HR=0.19; 95% CI: 0.13-0.26), time to castration resistance (HR=0.28; 95% CI: 0.22-0.36), time to first symptomatic skeletal event (HR=0.52; 95% CI: 0.33-0.80), time to initiation of new antineoplastics (HR=0.28; 95% CI: 0.20-0.40), with consistent results in the disease-volume subgroups.5 Over 65% of patients treated with enzalutamide + ADT achieved undetectable PSA level, which was significantly higher than those in the placebo group (p<0.001).4 Of note, over 80% of patients with low-volume disease achieved undetectable PSA level with enzalutamide + ADT.8
In a post-hoc analysis of ARCHES, the efficacy of enzalutamide + ADT in men with mHSPC was assessed by pattern of metastatic spread.6 Among the enrolled participants, the majority of patients had bone metastases only (n=513) or bone + LN metastases (n=351).6 Results demonstrated a higher efficacy of enzalutamide + ADT in patients with bone metastasis only (HR=0.33; 95% CI: 0.22-0.49) and bone + LN metastasis (HR=0.31; 95% CI: 0.21-0.47) when compared with LN metastasis only (HR=0.43; 95% CI: 0.16-1.20) or visceral metastasis (HR=0.94; 95% CI: 0.51-1.73) (figure 3).6
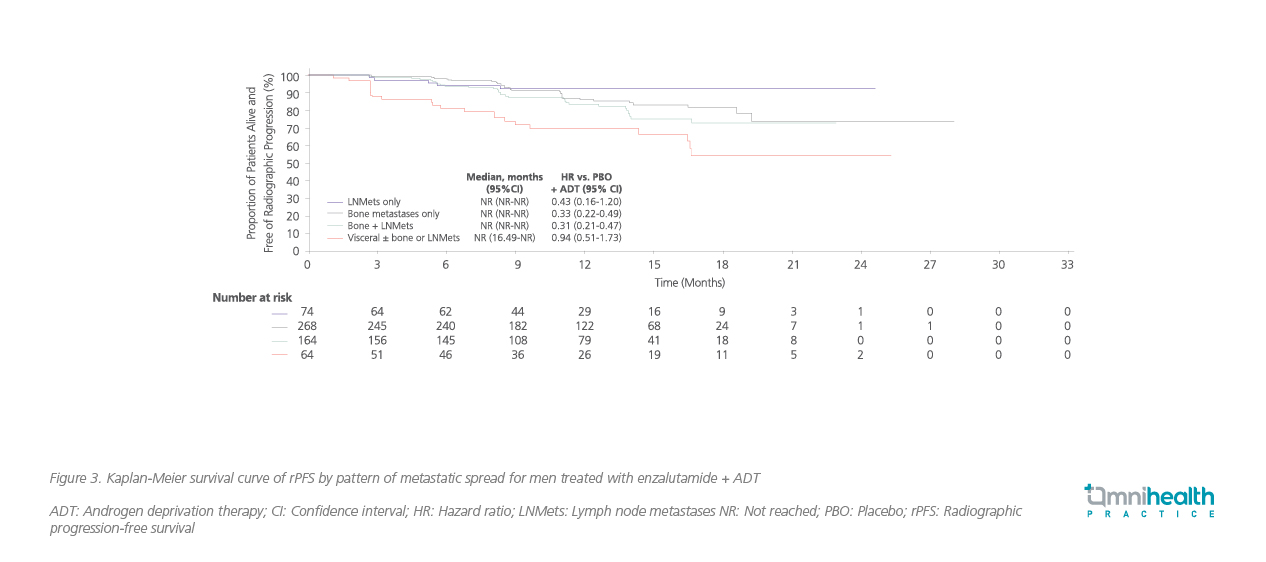
The ARCHES study was unblinded after reaching the primary endpoint of rPFS, 180 patients in the placebo arm (31.3%) crossed over to the enzalutamide group during the open-label period.7 In the long-term follow-up of ARCHES, with a median follow-up time of 44.6 months (data cutoff: May 28, 2021), the OS benefit of enzalutamide + ADT vs. placebo + ADT among mHSPC patients was demonstrated (HR=0.66; 95% CI: 0.53-0.81; p<0.0001) (figure 4).7,9 After adjusting the impact of crossover, the risk reduction in death with enzalutamide + ADT was 43% (HR=0.57; 95% CI: 0.45-0.70), having similar OS benefits with enzalutamide + ADT observed across all subgroups, including patients with low-volume disease (HR=0.56; 95% CI: 0.34-0.86).9
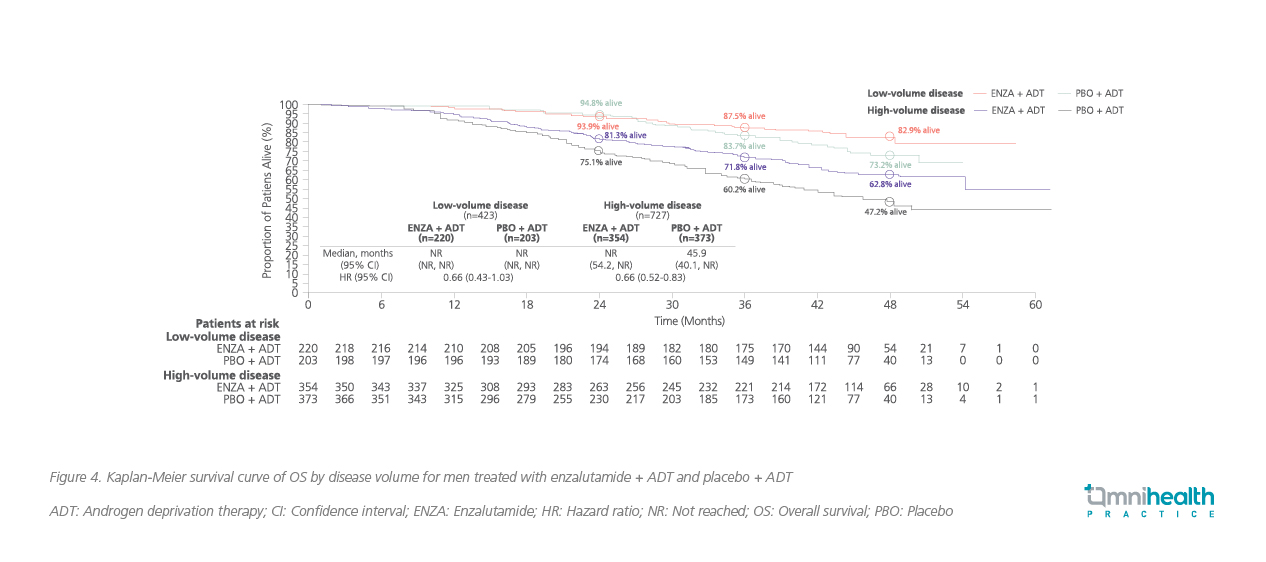
Nowadays, ADT alone should no longer be regarded as the SoC for patients with mHSPC. “Upfront AR agents, such as enzalutamide + ADT, have shown remarkable benefits in mHSPC patients, regardless of disease volume, and should be considered earlier for improving their clinical outcomes,” Dr. Poon concluded. Therefore, in the clinical case shared above, although the patient had low-volume disease, the combination of an AR agent with ADT was still associated with a significant OS benefit. Supported by the new evidence, adding AR agents to ADT has become a new SoC for the mHSPC management. In contrast, the role of upfront chemotherapy together with ADT in low-volume disease is quite controversial. The CHAARTED trial which included 790 men with mHSPC found that significant OS improvement was only observed in patients with high-volume disease.3 These findings were confirmed after longer follow-up (low-volume disease: HR=1.04; p=0.86; high-volume disease: HR=0.63; p<0.001).3
It is also worth to note that, in Hong Kong, about 540,000 people were chronically infected with hepatitis B in 2020.10 Additionally, thanks to the improved health care for chronic hepatitis B (CHB) patients, newborn prophylaxis and early childhood vaccination, the existing CHB population in Hong Kong has been aging rapidly.11 A local epidemiological study found that there has been an increasing prevalence of comorbidities among CHB patients, particularly those with hypertension and diabetes mellitus.11 Therefore, it is reasonable to take these factors into account when prescribing treatments for mHSPC patients. Enzalutamide is a viable treatment option for patients with hepatic impairment. Notably, dose adjustment is not necessary for patients with mild, moderate, or severe hepatic impairment.12
On the other hand, hypertension is common in patients treated with enzalutamide, therefore regular monitoring of patients’ BP might be required. In this case, the patient’s BP was slightly elevated after 2 years of enzalutamide treatment. “Although the patient’s BP was still within a normal range, I have discussed with him regarding the need for dose adjustment of the antihypertensive drug. Yet, the patient opined that the increase in BP was acceptable and insisted on monitoring the condition,” Dr. Poon said.
Conclusion
The treatment landscape of mHSPC continues to evolve, providing more treatment options for optimizing patient care and outcomes. Enzalutamide, in combination with ADT, has demonstrated superior efficacy vs. placebo + ADT, as well as a balanced and consistent safety profile in mHSPC patients, including those with low-volume disease, and has become a viable treatment option in this clinical setting.

