INDUSTRY ESSENTIALS
Navigating genetic testing in prostate cancer: An insight into the joint HKUA-HKSUO consensus recommendations
In 2019, prostate cancer (PC) was ranked the third most frequently diagnosed cancer among men in Hong Kong.1 Though genetic testing has not been previously considered a routine approach of PC patient care, recent sequencing studies revealed that about 11.8% of metastatic PC (mPC) patients carried inherited deoxyribonucleic acid (DNA) repair gene mutations based on germline testing and approximately 23% of castration-resistant PC (CRPC) patients presented with DNA repair pathway abnormalities through somatic testing.1 Consequently, international guidelines and consensus recommendations have expanded their criteria for PC genetic testing, now including not only patients with a family history of PC or related cancers, but also those with advanced CRPC and early-stage PC patients to determine the suitable active surveillance strategies.1
Despite that updated international guidelines are currently available, the implementation of these recommendations by the Asian populations remains challenging.1 Inter-ethnic variations in genetic mutation patterns, limited genetic counseling resources and the difficulty of selecting the most appropriate genetic testing panel could be the hurdles.1 To this end, the Hong Kong Urological Association (HKUA) and the Hong Kong Society of Uro-Oncology (HKSUO) have collaboratively developed a set of consensus guidelines, aiming to offer vital guidance on genetic testing for urologists, oncologists and pathologists when dealing with Asian PC patients.1
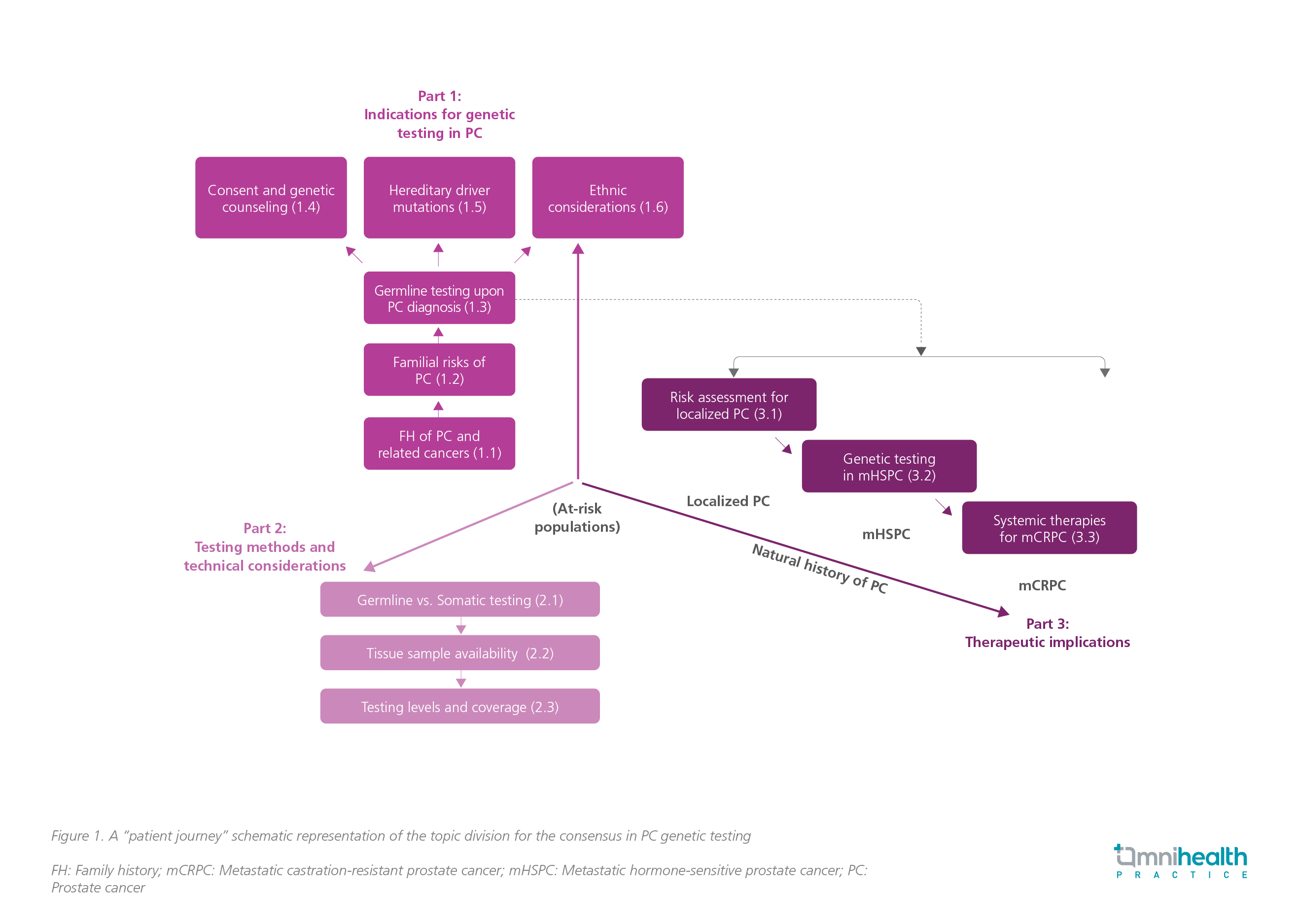
A structured and iterative approach was implemented by a joint consensus panel of 8 local specialists, consisting of 4 urologists, 3 oncologists and 1 pathologist, utilizing the Modified Delphi method.1 The panel adopted a “patient journey” approach and identified 3 key areas of debate: (1) The criteria for genetic testing in PC, (2) the testing methods and technical factors and (3) the treatment implications (figure 1).1 In total, 35 candidate statements were drafted for voting, with 31 statements ultimately being accepted.1