CASE REVIEW
A local case sharing: Dapagliflozin reduces proteinuria and slows disease progression in CKD patients
Dapagliflozin, a sodium-glucose co-transporter 2 (SGLT-2) inhibitor, has demonstrated improved renal outcomes in patients with chronic kidney disease (CKD), with or without type 2 diabetes (T2D), in the DAPA-CKD trial, providing an additional treatment option on top of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) for better management of CKD patients.1 In an interview with Omnihealth Practice, Dr. Tam, Chun-Hay shared a clinical case of an 89-year-old patient with stage 3 CKD without T2D, in whom dapagliflozin reduced proteinuria and stabilized his renal function with good tolerability. He also discussed the clinical data of dapagliflozin and provided some practical tips on its use to optimize treatment outcomes.
Background
CKD without T2D: A rising challenge
CKD has become one of the leading causes of death and suffering in the 21st century, affecting >10% of the global population.2 In 2017, an estimate of around 840 million individuals had CKD worldwide.2 Dr. Tam shared that non-diabetic CKD is common and about half of his CKD patients are non-diabetic. The common causes of non-diabetic CKD include hypertension (HTN) and glomerulonephritis (GN).4,5
Unmet needs among CKD patients and limitations of RAASi
Proteinuria is a well established and strong indicator of CKD progression.6 Traditionally, renin-angiotensin-aldosterone system (RAAS) inhibitors, including ACEis and ARBs, are known for their antiproteinuric effect and have been shown to delay CKD progression and reduce the risk of cardiovascular (CV) events and death.6 “However, some patients could not tolerate these conventional treatment options well due to hyperkalemia or low baseline blood pressure,” Dr. Tam reminded. For patients who remain proteinuric despite high doses of ACEi or ARB, the use of dual RAAS blockade could cause more hyperkalemia than a single RAAS antagonist alone.7 Thus, these patients are in need of a safe and effective alternative to control their proteinuria and delay CKD progression.
The emergence of dapagliflozin in CKD management
Dapagliflozin is an SGLT2 inhibitor that was initially developed as an oral glucose-lowering drug for the treatment of T2D.8 In the DECLARE-TIMI 58 trial, it was found that dapagliflozin reduced renal specific outcome and albuminuria in T2D patients.9 These findings aroused researchers’ interest in evaluating the effect of dapagliflozin on long-term kidney outcomes in CKD patients. As such, the DAPA-CKD trial, a randomized, double-blind, placebo-controlled, multicenter trial, was conducted to extend these findings to CKD patients with or without T2D.1 In this trial, a total of 4,304 CKD patients, regardless of the T2D status, with an estimated glomerular filtration rate (eGFR) of 25-75mL/min/1.73m2 and a urine albumin-to-creatinine ratio (UACR) of 200-5,000mg/g were enrolled.1 The patients had received stable doses of ACEi/ARB.1 After a median follow-up of 2.4 years, results showed that dapagliflozin significantly reduced the risk of primary composite outcome of a sustained ≥50% decline in eGFR, end-stage kidney disease (ESKD), or death from renal or CV causes vs. placebo (HR=0.61; 95% CI: 0.51-0.72; p<0.001).1
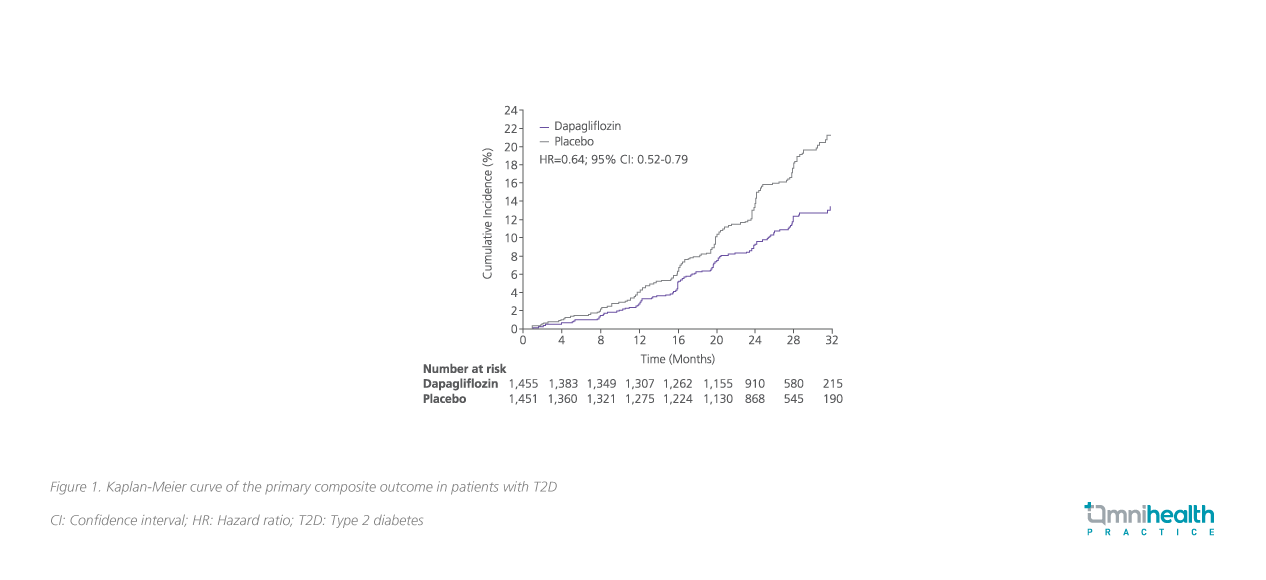
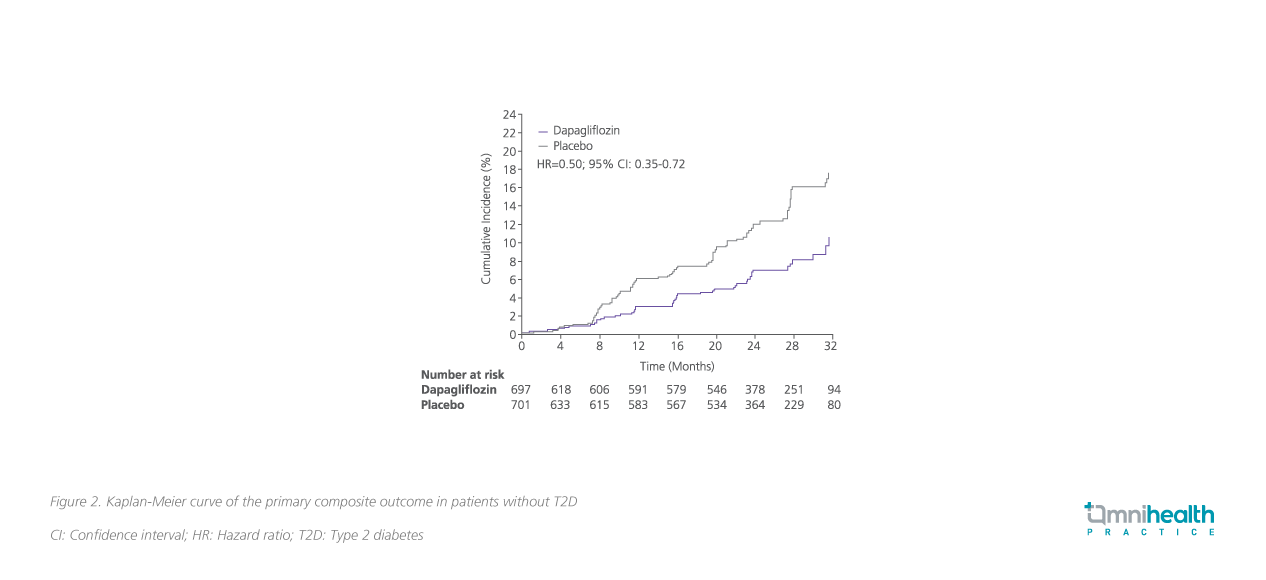
Furthermore, a subgroup analysis showed that dapagliflozin significantly reduced the primary composite outcome in CKD patients with (HR=0.64; 95% CI: 0.52-0.79) or without T2D (HR=0.50; 95% CI: 0.35-0.72) (pInteraction=0.24) (figure 1 and 2), showing consistent efficacy regardless of the T2D status.10
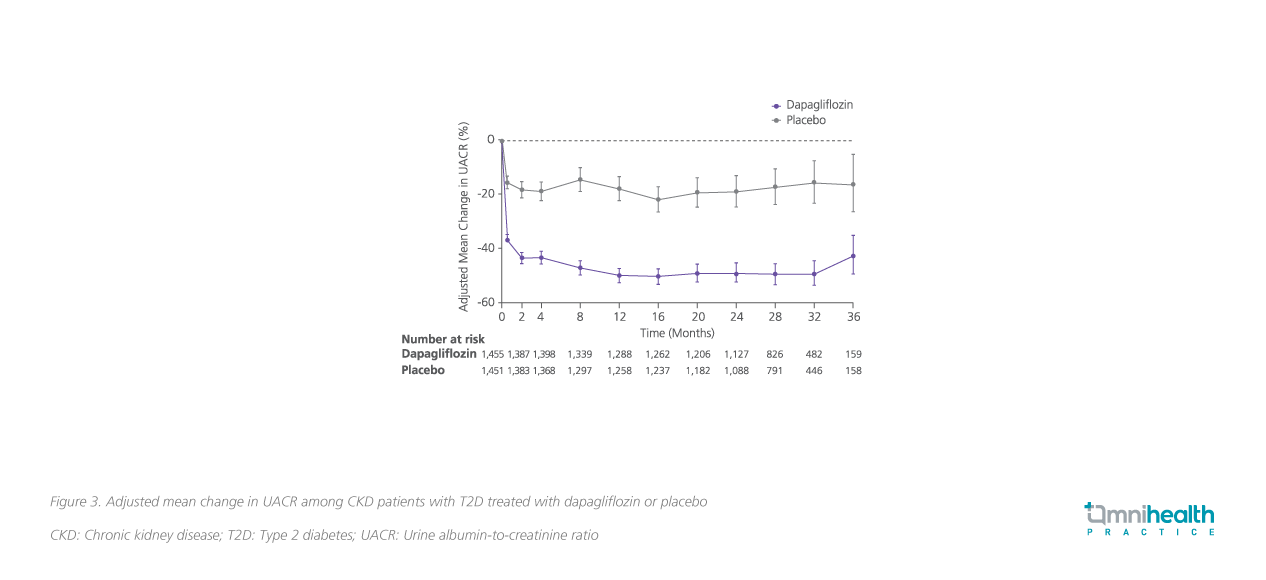
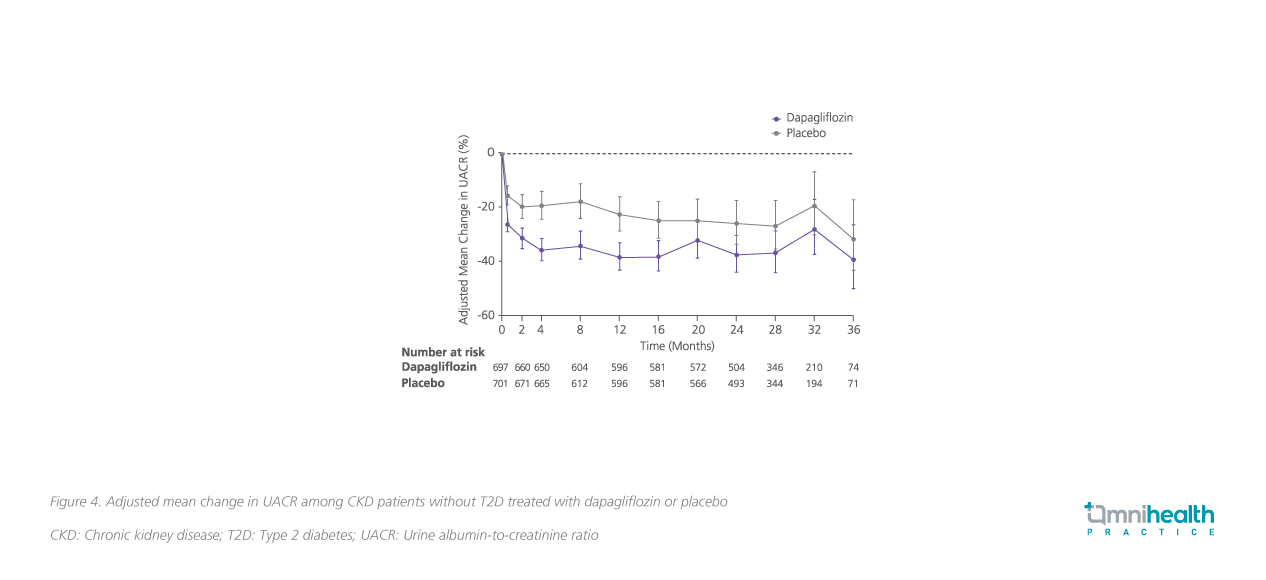
In the UACR subgroup analysis, dapagliflozin was shown to significantly reduce UACR by 29.3% (95% CI: -33.1 to -25.2; p<0.0001) vs. placebo among CKD patients, regardless of the T2D status.8 Notably, treatment with dapagliflozin resulted in 35.1% reduction in UACR in T2D patients (95% CI: -39.4 to -30.6%; p<0.0001), while for patients without T2D, the decrease in UACR was found to be 14.8% (95% CI: -5.9 to -22.9%; p=0.0016) (figure 3 and 4).8
Overall, dapagliflozin was well tolerated by CKD patients with or without T2D.1 The incidence of adverse events (AEs) and serious adverse events (SAEs) were similar in the dapagliflozin and placebo groups.1 Diabetic ketoacidosis and severe hypoglycemia were not observed in participants without T2D.1
Based on the positive trial data, Dr. Tam adopted dapagliflozin in his practice and shared a clinical case on the antiproteinuric and renoprotective effects of dapagliflozin in a local, 89-year-old patient with CKD.
Case sharing
An 89-year-old, non-diabetic male with baseline hypertension and benign prostate hyperplasia (BPH) was diagnosed with stage 3 CKD in 2016. According to the medical records, his serum creatinine level was stable at around 90μmol/L between 2011 to 2016. However, his conditions started deteriorating and his serum creatinine level increased to 140-160μmol/L in the next 4-5 years. He was also found to excrete 1-2g/L urine protein a day.
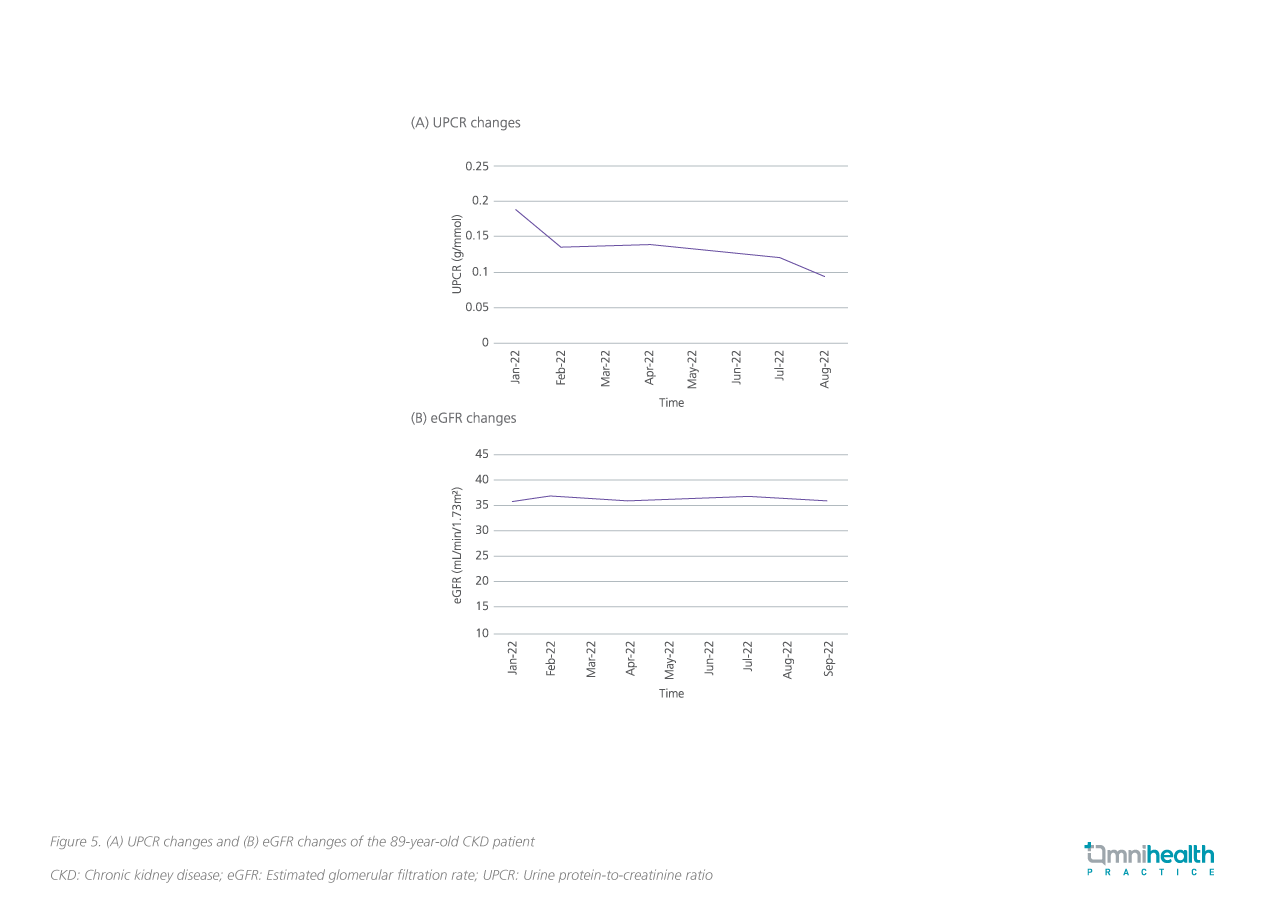
When the patient was referred to Dr. Tam in 2021, the patient was already on high-dose dual RAAS inhibition. Although this combination was well tolerated by the patient, Dr. Tam was concerned about the elevated risk of hyperkalemia and acute kidney injury (AKI) associated with the dual RAAS blockade, and therefore discontinued ACEi from the regimen. In December 2021, Dr. Tam added dapagliflozin 10mg daily to treat the patient’s proteinuria and preserve his renal function. The patient responded well to dapagliflozin, and his proteinuria reduced from 0.188g/mmol to 0.095g/mmol during the first 9 months of dapagliflozin treatment (figure 5). In addition, his renal deterioration was also halted, with eGFR stabilized at around 36mL/min/1.73m2 (figure 5). The hemoglobin A1c (HbA1c) and blood pressure (BP) of the patient also remained stable throughout the year of treatment with dapagliflozin.
Discussion
Dr. Tam reckoned that it was difficult to manage CKD in the past due to the limited treatment options. It was even more challenging when the patients had CKD of unknown etiology and refused renal biopsy. For this patient, dual RAAS blockade with ACEi and ARB was previously utilized to control proteinuria, but in vain. An additional treatment option that can effectively reduce proteinuria with good safety profile has long been sought.
At first, Dr. Tam hesitated to prescribe dapagliflozin since the patient did not have T2D. He was uncertain if dapagliflozin would lead to hypoglycemia, considering the glucose-lowering effect of the drug. Yet, the patient did not report any episodes of hypoglycemia which showed that the renal protective effect of dapagliflozin is independent of glucose-lowering.
On the other hand, data have shown that dapagliflozin could lead to an initial dip in eGFR in the first few weeks of treatment owing to its renal mechanism of action, but in this case, there was no initial dip in eGFR. Dr. Tam concluded that despite the clinical data, the initial eGFR dip might not necessarily happen and he suggested monitoring patients’ renal function regularly every 3 months.
Conclusion
In summary, “although dapagliflozin is originally an anti-diabetic drug, it can benefit CKD patients even if they do not have T2D,” Dr. Tam said. Clinical trials and real-world experiences showed that dapagliflozin is effective in reducing proteinuria and slowing CKD progression in patients with or without T2D. In addition, dapagliflozin was safe and well tolerated by elderly CKD patients with no hypoglycemia observed especially in non-diabetic CKD patients in his practice. Dr. Tam recommended that clinicians could improve the outcomes of their CKD patients by considering early initiation of dapagliflozin as adjunct to first-line ACEi/ARB treatment, or as an alternative treatment option when ACEi/ARB is not suitable.
This is an independent editorial article, published and distributed through unrestricted educational support from AstraZeneca Hong Kong Limited, for the purpose of continuing medical education only. The views expressed in this publication reflect the experience and/or opinion of the author(s) and are not necessarily those of editors, publisher and sponsor(s). Because of rapid advances in medicine, independent verification of clinical diagnoses, medical suitability and dosage should be made before treatment prescription. The appearance of advertisement, if any, has no influence on editorial content or presentation and does not imply the endorsement of products by the publication, or its authors and editors.
- Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383;1436-1446.
- Kovesdy CP, et al. Epidemiology of chronic kidney disease: An update 2022. Kidney International Supplements. 2022;12:7-11.
- Plantinga LC, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673-682.
- Horowitz B, et al. Epidemiology of hypertension in CKD. Advances in Chronic Kidney Disease. 2015;22(2):88-95.
- Kazi AM, et al. Glomerulonephritis. StatPearls Publishing. Jan 2022.
- Gorriz JL, et al. Proteinuria: Detection and role in native renal disease progression. Transplantation Reviews. 2012;26:3-13.
- Pichler RH, et al. Dual renin-angiotensin-aldosterone system blockade for diabetic kidney disease. Curr Diab Rep. 2010;10(4):297-305.
- Jongs N, et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:755-766.
- Mosenzon O, et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44:1805-1815.
- Wheeler DC, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol.2021;9(1):22-31.

