EXPERT INSIGHT
BNT162b2 boosters and beyond: Strategies to overcome waning immune responses against omicron variant
During the fifth wave of Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [B.1.1.529 (Omicron), predominant] infected over a million people and claimed more than 9,000 lives in Hong Kong.1 Elderly people aged ≥60 years were the most vulnerable population and accounted for ≥95% of the total death cases.1 Among the deceased, most were unvaccinated (>70%), or had received only 1 dose of COVID-19 vaccine, and/or were with known chronic diseases.1 Even for people who have received 2 doses of vaccines, increasing evidence showed that protection against severe COVID-19 would be reduced due to the waning immune responses against the Omicron variant.2 As such, in an interview with Omnihealth Practice, Professor Hung, Fan-Ngai Ivan, addressed the rising concern about Omicron and presented the latest data showing that BNT162b2 boosters are still highly effective against the new threat. He also provided some updates on the development of new Omicron-adapted vaccines for conferring better protection to people.
Increased immune escape of Omicron and waning NAb (neutralizing antibody) against COVID-19
The increased escape of the Omicron variant from neutralizing activities through mutations in the receptor-binding domain of spike protein prompted concerns about the reduced protection among the vaccinated population.3 A German study found that the Omicron-NAb titers, when compared with the wild-type virus, were reduced by more than 22-fold after 2 doses of BNT162b2.4 A similar result was obtained by a group of microbiologists from The University of Hong Kong, proving that 2 doses of BNT162b2 were inadequate in preventing the Omicron infection, and that people who have received COVID-19 vaccines and those who have recovered from COVID-19 were also at an increased risk of having a breakthrough infection or reinfection.5
Some studies revealed that the level of T cell response was associated with the degree of disease severity of COVID-19.6 Patients with robust T cell responses to SARS-CoV-2 infection were more likely to have asymptomatic or mildly symptomatic disease, whereas those with dysfunctional T cell responses tended to suffer from more severe illnesses.6 Similar to the impact on humoral response, T cell response of two doses of BNT162b2 was also reduced by the spike mutations of Omicron, though to a much lesser extent.3 The frequency of tissue necrosis factor (TNF)-producing CD4+ T cells and interleukin-2 (IL-2)-producing CD4+ T cells against the Omicron spike protein was reduced by 14% (p<0.005) and 21% (p<0.001) compared to the wild-type spike protein, respectively.3
The real-world effectiveness of two doses of BNT162b2 against symptomatic Omicron infection was shown in a large case-control study in the United Kingdom (UK) in which 887,774 people were infected with Omicron and 1,575,621 test-negative controls were included for analysis.2 At 2-4 weeks post-vaccination, a high vaccine effectiveness (VE) of 65.5% (95% CI: 63.9-67.0) was achieved.2 However, it dropped remarkably to 8.8% (95% CI: 7.0-10.5) after ≥25 weeks of completing the 2-dose series.2
Restoring high vaccine protection against Omicron with BNT162b2 booster
Despite the reduction in Omicron-NAb titers and T cell responses with 2 doses of BNT162b2, an additional dose of mRNA vaccine managed to significantly boost the NAb titers and maintain high T cell responses against the Omicron variant.3,4 A third dose of BNT162b2 was shown to increase Omicron-NAb titers by 23-fold compared with the levels after receiving 2 doses, which was similar to the levels achieved by 2 doses of BNT162b2 against the wild-type virus (figure 1).4 The frequencies of TNF-producing CD4+ T cells and IL-2-producing CD4+ T cells against the Omicron spike protein were also raised significantly by the BNT162b2 booster dose to levels similar to those achieved by 2 doses of BNT162b2 against the wild-type spike protein.3
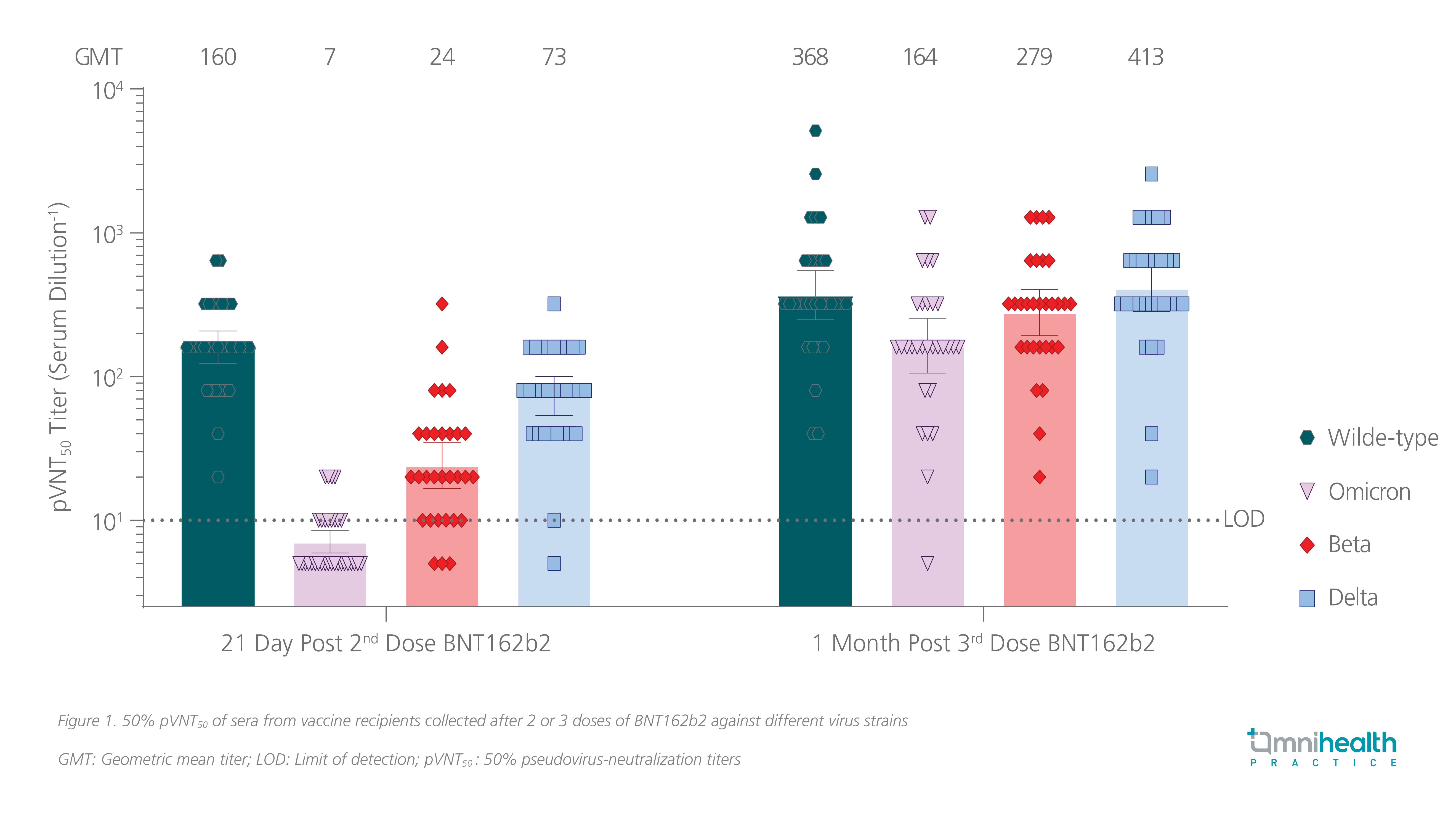
The vaccine efficacy of BNT162b2 booster (the third dose) against symptomatic COVID-19 in the general population was evaluated in a randomized, placebo-controlled, phase 3 trial which included 10,125 people having received 2 doses of BNT162b2 at least 6 months earlier.7 Half of the participants were given a third dose of BNT162b2 and were followed up for a median of 2.5 months.7 A relative vaccine efficacy of 95.3% (95% CI: 89.5-98.3) was attained, and no new safety signal was identified.7
In the real-world setting, a large case-control study in the UK which included 2,663,549 people confirmed the high effectiveness of BNT162b2 against the Omicron variant.2 Among people who had completed the 2-dose series, vaccine effectiveness of 67.2% (95% CI: 66.5-67.8) at 2-4 weeks was achieved after receiving a BNT162b2 booster.2
Protect the elderly against severe disease and death with a fourth dose
Contracting SARS-CoV-2 infections could result in heterogeneous clinical presentations, ranging from asymptomatic cases to severe diseases and death.8 Certain risk factors have been identified to predict the elevated risk of severe illness and mortality of the infected individuals.8 Among them, advanced age was shown to be strongly associated with worse adverse outcomes.8 People who were aged >75 years were shown to have a 1.65-fold higher risk of suffering from severe conditions (OR=2.65; 95% CI: 1.81-3.90) and a 4.57-fold increased risk of death (OR=5.57; 95% CI: 3.10-10.00) compared with the younger population.8 Male sex (OR=2.05; 95 % CI: 1.39-3.04) and active cancer (OR=1.46; 95% CI: 1.04-2.04) were other important factors contributing to severe outcomes in COVID-19.8
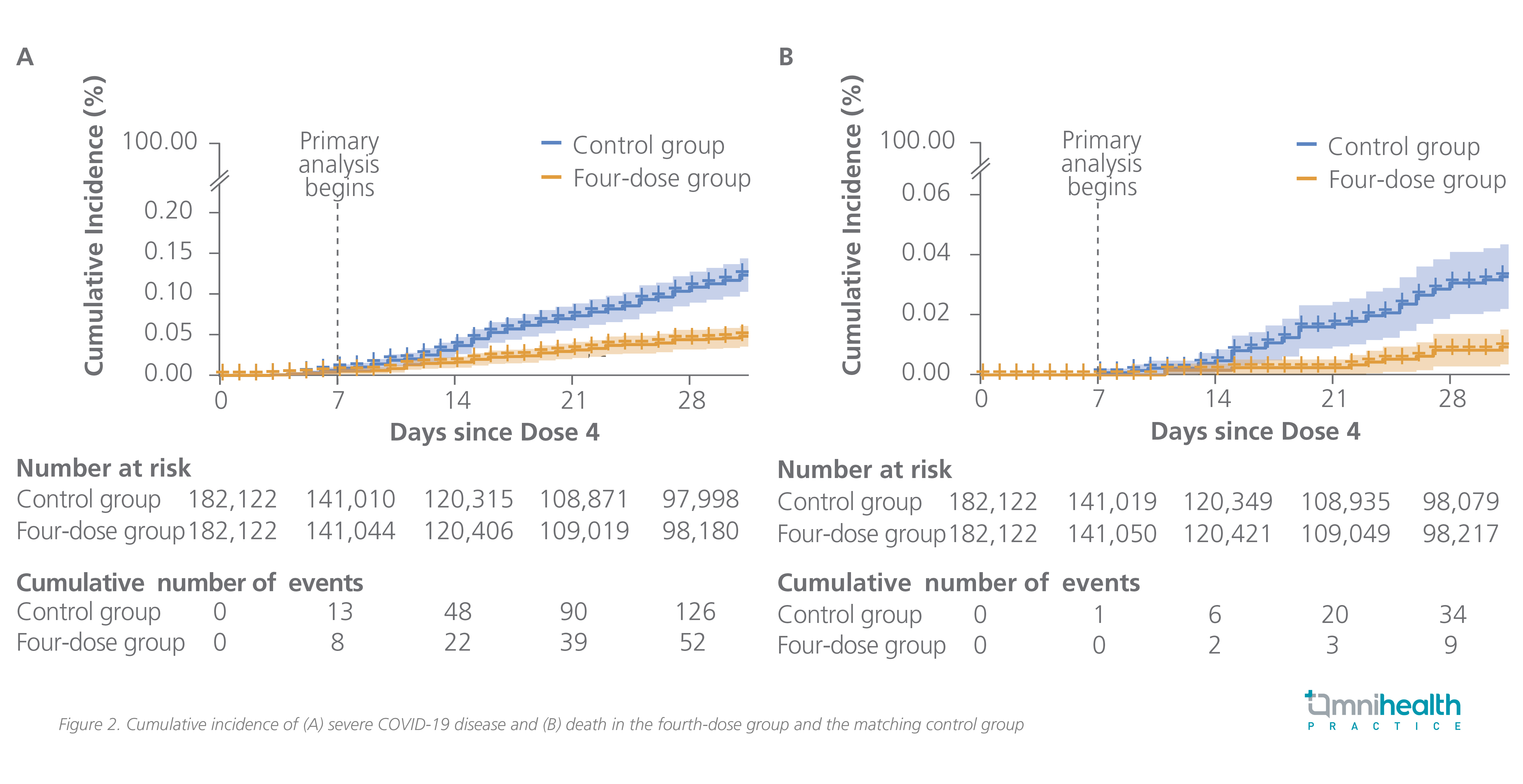
Considering the elevated risk of severe diseases and mortality among the elderly people and the waning protection against Omicron, it is imperative to step up the preventive measures for the older population.2,8 The vaccine effectiveness of the BNT162b2 second booster (i.e. the fourth dose) against COVID-19-related outcomes caused by the Omicron variant among people aged ≥60 years or older were assessed in an Israeli real-world study.9 The included subjects had received a third dose of BNT162b2 at least 4 months earlier and were given the fourth dose of mRNA vaccine.9 The relative vaccine effectiveness of the second booster of BNT162b2 after 14-30 days of vaccination was found to be 52% (95% CI: 49-54) against the Omicron infection, 61% (95% CI: 58-64) against symptomatic disease, 72% (95% CI: 62-79) against COVID-related hospitalization, 64% (95% CI: 48-77) against severe COVID-19, and 76% (95% CI: 48-91) against COVID-19-related death (figure 2).9
The fourth dose of BNT162b2 for immunocompromised people
Immunocompromised patients were recognized early in the COVID-19 pandemic as a vulnerable group of severe diseases and mortality because of the possible suppression or over-activation of the immune system due to the primary disease or concurrent treatment.10,11 A prospective, open-label trial found that a significantly lower proportion of immunocompromised patients were seroconverted after vaccination compared with immunocompetent people (72.2% vs. 100%; p=0.004).10 The lowest seroconversion rates were found among patients with solid organ transplant (43.4%) and chronic lymphocytic leukemia (63.3%) with observed negative impact of mycophenolate mofetil and ibrutinib, respectively.10
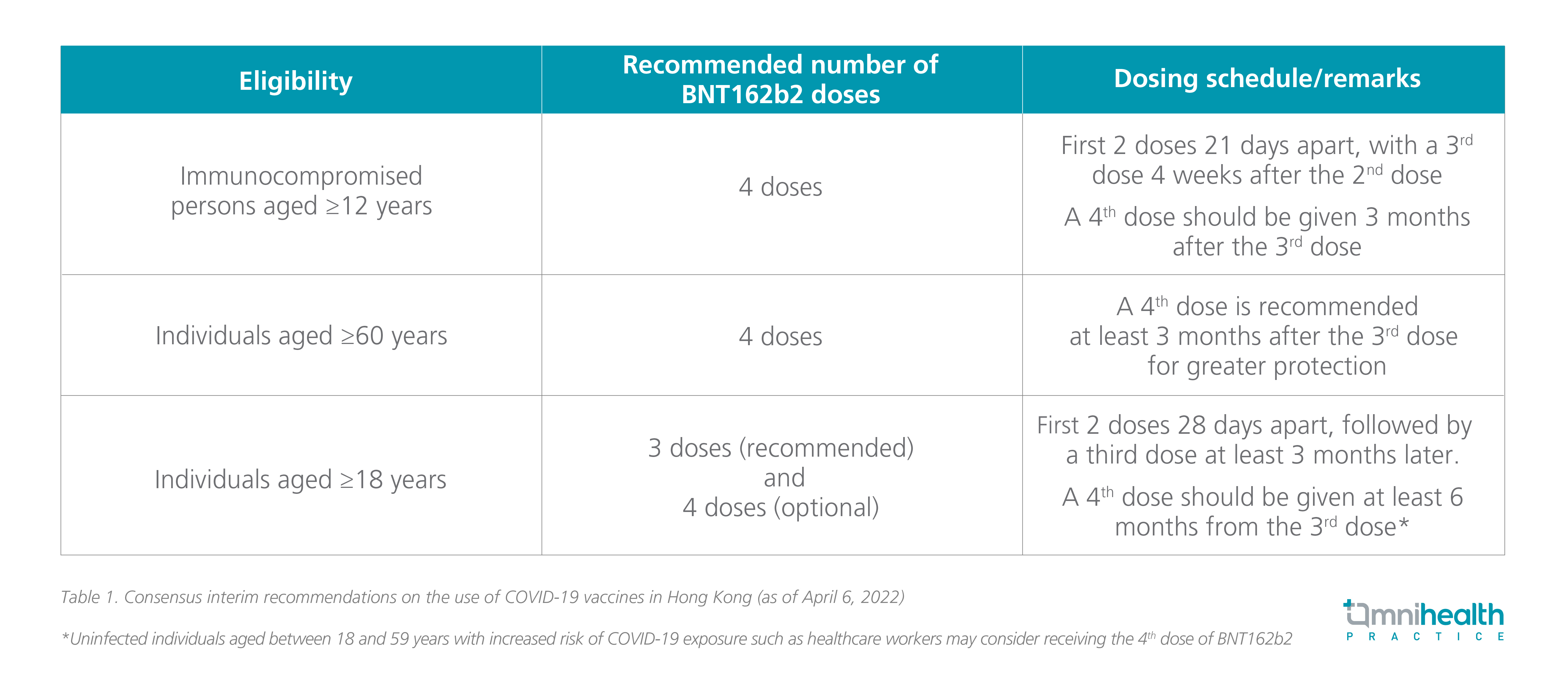
In light of the impaired seroconversion among the immunocompromised patients, the Joint Scientific Committee with the Chief Executive’s expert advisory panel (JSC-EAP) recommends the fourth dose of BNT162b2 for immunocompromised individuals aged ≥12 years and elderly aged 60 or above for better protection (table 1).12
A heterologous boosting vaccine strategy for better immune response against the Omicron variant
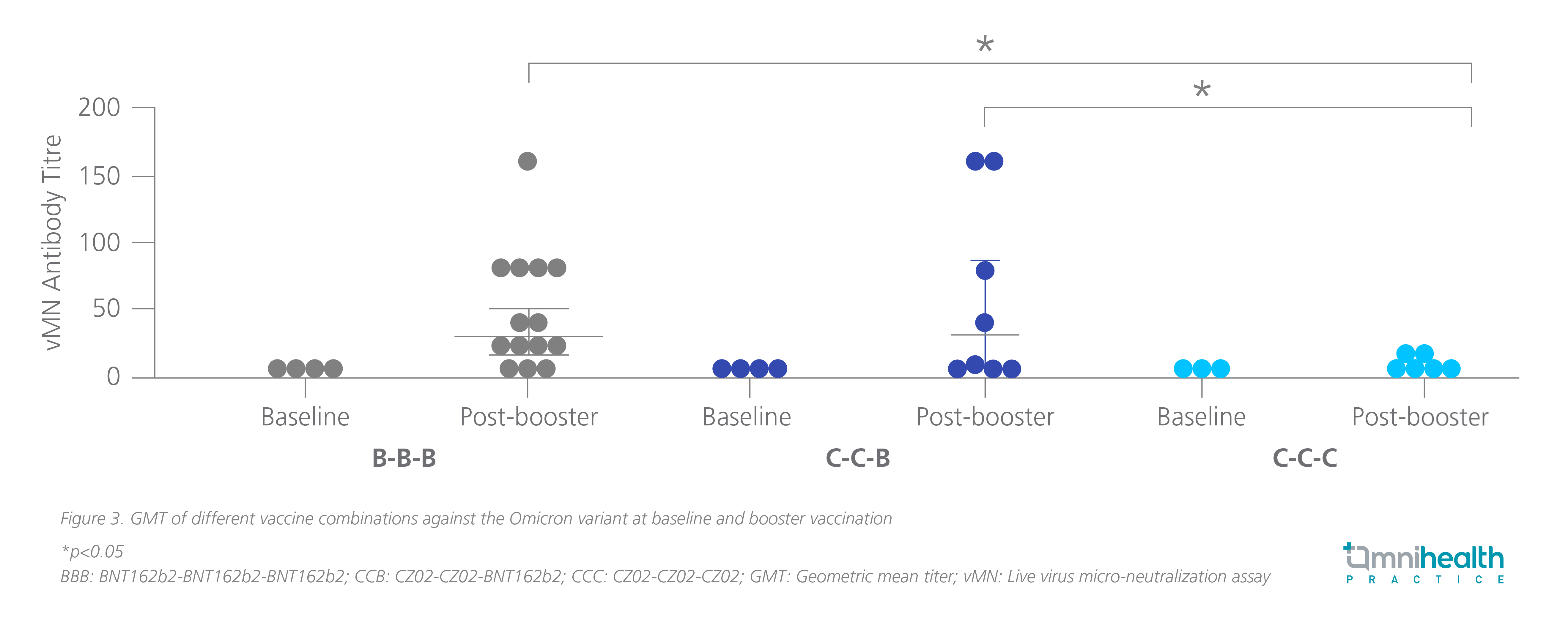
In Hong Kong, BNT162b2 and CZ02 were adopted for large-scale COVID-19 vaccination, and people can opt for either one for boosting. Yet, the immune response of BNT162b2 and CZ02 boosters against the Omicron variant was unknown.13 Two local studies were therefore conducted to evaluate the immunogenicity of the two vaccine boosters among people who had previously received 2 doses of COVID-19 vaccines.13,14 As expected, the increase in Omicron-NAb titers was significantly higher in participants who were given a BNT162b2 booster than those administered with CZ02 booster (figure 3).13 The geometric mean titers of anti-Spike IgG [1,400; (95% CI: 1,035-1,894) vs. 217.8 (95% CI: 152.7-310.5) and anti-RBD IgG [683.3 (95% CI: 498.4-936.9) vs. 17.8 (95% CI: 4.1-76.7)] were also significantly higher with BNT162b2 booster when compared with CZ02, indicating a more prominent T cell response with BNT162b2 booster.14
Regarding safety, the heterologous boosting vaccine strategy was well tolerated, with pain at the injection site being the most reported adverse events (AEs).13 No serious AEs were reported.13
COVID-19 Vaccination among convalescent patients
Although some observational studies have shown that prior SARS-CoV-2 infections could elicit immune response and provide recovered COVID-19 patients with a certain extent of protection against recurrent infection, the duration of natural immunity against Omicron infection remains uncertain.15,16 A recent population-level observational study in Italy (N=1,293,941) showed that the reinfection rates among people previously infected (n=119,266) with SARS-CoV-2 were 6.1% after 277days and 6.7% after 18-22 months from the primary infection.16 The risk of reinfection with Omicron was significantly higher among unvaccinated people and females.16 In addition, a study from The University of Hong Kong found that among the included non-vaccinated patients who were previously infected with SARS-CoV-2 in 2020 and early 2021, only 12.3% of them had detectable NAb against Omicron.15After a single dose of BNT162b2 and a single dose of CZ02, the Omicron-seropositive rates were significantly increased to 90.6% (p<0.001) and 36.7% (p=0.0115), respectively.17 Of note, two doses were required for CZ02 to increase the Omicron-seropositive rates to 85.7% (p=0.0045).17 The findings highlight the need for convalescent people to receive COVID-19 vaccination.
Besides, the vaccine effectiveness of BNT162b2 among the recovered patients to prevent reinfection was evaluated in a retrospective cohort study (N=149,032) in Israel.15 The reinfection rate among people who received the subsequent BNT162b2 vaccination (N=83,356) was 2.46 cases/100,000 persons/day vs. 10.21 cases/100,000 persons/day for the unvaccinated patients.15 The vaccine effectiveness of BNT162b2 for preventing reinfection was estimated to be 82% (95% CI: 80-84) among patients aged between 16-64 years and 60% (95% CI: 36-76) among those aged ≥65 years.15 A single dose of BNT162b2 was found to be as effective as 2 doses.15
Next-generation Omicron-adapted COVID-19 vaccines
Omicron is the most distinct SARS-CoV-2 variant to date which displays multiple amino acid alterations in the neutralizing antibody sites of the spike protein and effectively evades the host immunity.18 Encouragingly, Omicron breakthrough infection in BNT162b2 vaccinated individuals was recently reported to elicit strong neutralizing activity not only against Omicron but also broadly against other previous variants and a robust B cell recall response in which preformed B cells could recognize epitopes shared by different variants, rather than specifically to the epitopes of Omicron.18 This finding suggests that Omicron-adapted vaccination after BNT162b2 could provide cross-strain immunity to the vaccinated individuals and prompts the development of the next-generation COVID-19 vaccine.19
In early 2022, a bivalent wild-type/Omicron-adapted and a monovalent Omicron-adapted vaccines have been in development, and a study was initiated to evaluate the safety, tolerability, and immunogenicity of the Omicron-adapted vaccines among people aged between 18 and 55 years who were unvaccinated or had received 2-3 doses of BNT162b2 (figure 4).20 The study is still ongoing, and the data readout will be in the coming months.20
There is no evidence and sign that mutations of SARS-CoV-2 will stop at Omicron. No doubt, continuous efforts to develop novel vaccines are warranted to overcome the future challenges of the upcoming SARS-CoV-2 variants. Strategies with prospect include the development of a vaccine which targets multiple SARS-CoV-2 variants (multivalent), a vaccine with enhanced T cell response, and ultimately, a vaccine which could cover all possible variants of SARS-CoV-2.18
Conclusion
Vaccination remains an effective measure to protect the local population from severe COVID-19 and its associated death. It is highly encouraged that all eligible individuals, especially the elderly and immunocompromised people, should receive the recommended doses of COVID-19 vaccines for boosting their immunity against the virus. Given the higher immune response compared with CZ02 and the high vaccine effectiveness, BNT162b2 is the preferred option as the vaccine boosters and the primary vaccine series for those who have prior COVID-19. The development of an Omicron-adapted vaccine to address the issue of rapidly waning protection against Omicron is underway. It is hoped that new generation vaccines, once available, could provide a stronger, long-lasting, cross-variant protection and save people the hassles of repeated boosters.

