The arrival of the JN.1 sublineage of the Coronavirus disease 2019 (COVID-19) Omicron variant, shows a major antigenic shift from initial variants.1 As JN.1 evolves, continuous surveillance, vaccination initiatives, and diligent adherence to preventive measures are vital for minimizing its potential impact on global public health.1 During a recent symposium, Professor Hung, Fan-Ngai Ivan from the University of Hong Kong emphasized the importance of randomized controlled trials and real-world data in evaluating vaccine safety and effectiveness across diverse populations, including children, the elderly and immunocompromised individuals. He highlighted that the BNT162b2 JN.1-adapted messenger ribonucleic acid (mRNA) vaccine is the only mRNA vaccine with comprehensive data available, reinforcing the necessity of ongoing monitoring of vaccine performance and the potential impact of new variants on public health. His presentation underscored the significant advancements in vaccine development and the essential role of vaccination in reducing severe outcomes from COVID-19.
Unraveling the JN.1 threat: A new era in COVID-19 evolution
Despite the pandemic being declared over, COVID-19 remains a major public health challenge globally, with a 30% increase in weekly infections and a 26% rise in deaths.2 The burden of COVID-19 continues to be substantial, with hospitalization and death rates comparable to those of influenza.3 For individuals aged 65 and older, hospitalization rates are notably higher, with an in-hospital death rate of around 4.6% compared to 2.6% for influenza.3 Hospitalizations occur year-round, peaking during colder months.3 Vaccination has reportedly saved 1.6 million lives in the World Health Organization (WHO) European Region, primarily among older adults.4
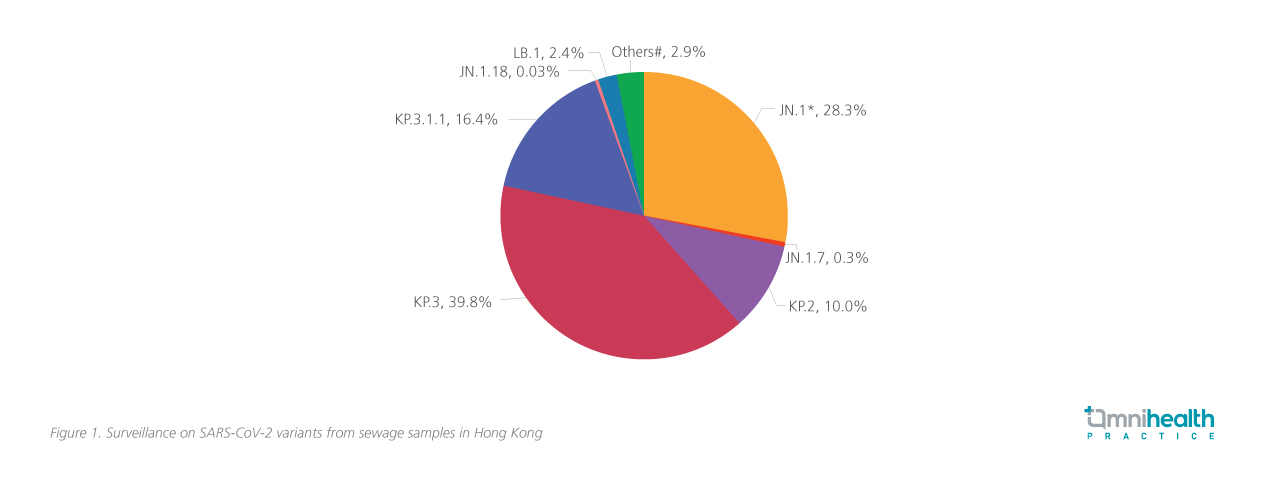
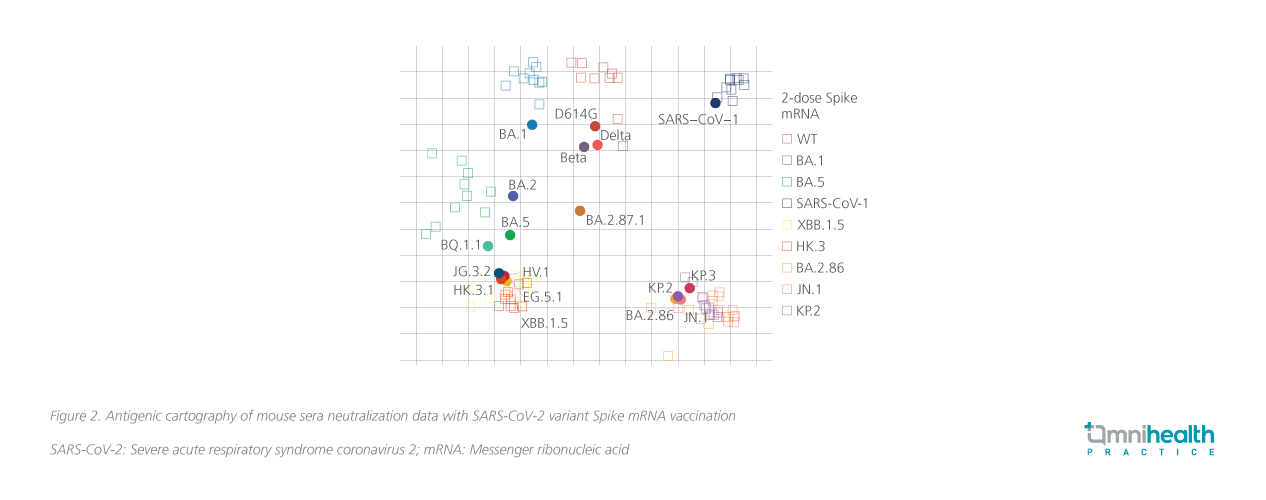
The JN.1 sublineages, which emerged in April 2024, have quickly become the dominant strains worldwide, including in Hong Kong (figure 1).5,6 This variant exhibits significant changes in the receptor-binding domain and the spike protein compared to XBB.1.5 (figure 2).1,7 Unlike the earlier XBB.1.5 strain, JN.1 variants, particularly due to the L455S mutation, demonstrate enhanced immune evasion and transmissibility, escaping neutralization by antibodies from previous infections and vaccinations, facilitating its rapid global spread.1 The rapid spread has also incurred significant economic costs.8 A global study of 148 countries found that COVID-19 vaccinations significantly mitigated economic losses — saving an estimated $5.2 trillion compared to a scenario without vaccines whilst also averting 1.7 billion infections and 4.1 million deaths, with benefit-cost ratios of 13.9 for all vaccines.8
![]()
![]()
Prof. Hung emphasized that an effective COVID-19 vaccine must demonstrate consistent efficacy in clinical trials and undergo continuous safety monitoring across all levels. It should also prove effective in diverse populations, including children and the elderly, with real-world evidence that reflects local demographics and health conditions. He noted that the BNT162b2 mRNA vaccine is currently the only mRNA formulation meeting these criteria.
Defend against JN.1: Long-term efficacy of the mRNA vaccine from clinical studies
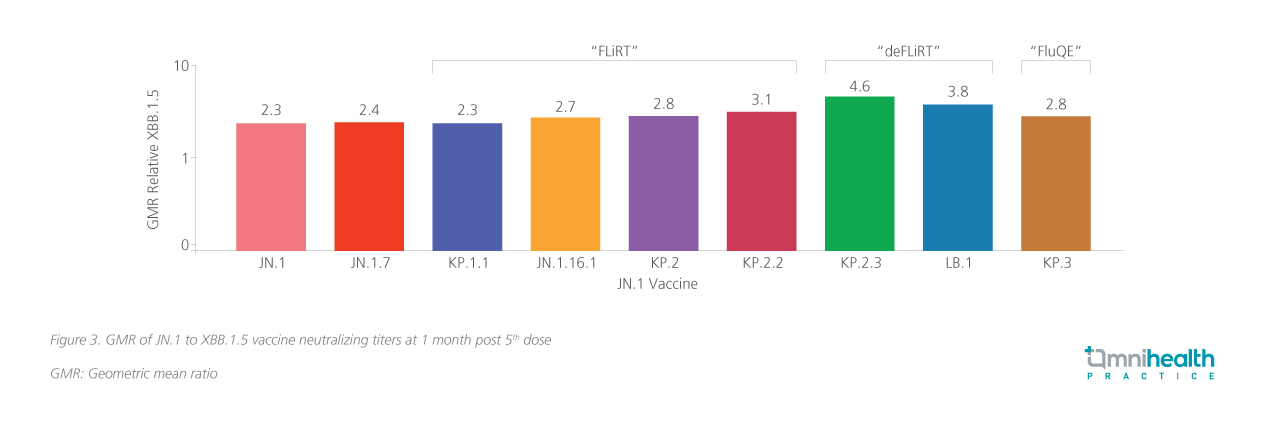
The BNT162b2 JN.1-adapted mRNA vaccine has demonstrated its efficacy against newer circulating variants.9 These studies indicate that this monovalent vaccine offers strong efficacy and cross-neutralization against multiple strains.9 Additional study involving healthcare workers further demonstrate significant boosts in neutralizing antibody levels – 2.2-fold against JN.1, 3.8-fold against KP.2, and 4.9-fold against LB.1.10 In vaccine-experienced populations, the BNT162b2 vaccine further demonstrated significant boosts in neutralizing antibody levels – 2.3-fold against JN.1, 2.8-fold against KP.2, 4.6-fold against KP.2.3, and 3.8-fold against LB.1 (figure 3).9 It has also been shown to boost neutralization of KP.3.1.1pp (geometric mean titer [GMT] reduction by 1.9-fold) and XEC (GMT reduction by 2.9-fold).11
![]()
Consistent safety profile from all levels and insights into myopericarditis
Not second to efficacy, safety is also important and is continuously monitored through comprehensive reporting from local level to regional and global levels. The updated version of BNT162b2 mRNA vaccine has a safety profile similar to that of the original and bivalent vaccines, with no new safety signals detected. Most adverse events reported within one month of vaccination were mild to moderate.12 A retrospective study in Hong Kong found that thromboembolism was the most frequently reported adverse event among recipients of both inactivated vaccine and BNT162b2 mRNA vaccine.13 Incidence rates for thromboembolism were higher for the first dose of inactivated vaccines (431 per 100,000 person-years) compared to BNT162b2 mRNA (290 per 100,000 person-years).13 The study also showed that the first dose of the inactivated vaccine showed a higher incidence of Bell's palsy, overall rates of adverse events of special interest and mortality were comparable between both vaccine types after both doses.13
A recent meta-analysis found that the incidence of myopericarditis related to COVID-19 infection is approximately 42 times higher than that associated with receiving the COVID-19 vaccine.14 There was no significant difference in the incidence of myopericarditis between the two mRNA vaccines and other vaccines (41.6 cases per million, 95% CI: 26.4-60.0) compared to the combination of BNT and other vaccines (27.4 cases per million, 95% CI: 5.6-64.0; p=0.45).14 Most cases are mild and self-limiting, with no significant long-term effects reported.15 Prof. Hung highlighted that “management strategies for myopericarditis include careful symptom monitoring, appropriate use of anti-inflammatory medications, and recommendations for longer dose interval to minimize risk. Adjusting injection site protocols, such as alternating arms for subsequent doses, can also be beneficial. Ongoing safety monitoring reinforces confidence in the vaccine's safety profile, ensuring that the benefits of vaccination far outweigh the risks.”
Real-world impact: Evidence of BNT162b2 mRNA vaccine effectiveness
Real-world data provide crucial insights into the vaccine’s effectiveness in practice. The vaccine effectiveness (VE) of the XBB.1.5-adapted vaccine against severe COVID-19 has been demonstrated internationally, particularly during the circulation of the XBB and JN.1 variants. In the Netherlands, the VE among previously vaccinated adults aged 60 years and older was estimated at 71% against hospitalization and 73% against intensive care unit (ICU) admissions.16 In the United States, vaccinated adults experienced a 58% reduction in the risk of emergency department or urgent care visits, as well as a 58% reduction in the risk of outpatient visits for COVID-19, observed after a median of 30 days following the XBB.1.5-adapted dose compared to those who did not receive the vaccine.17
Notably, the BNT162b2 mRNA vaccine is the only mRNA vaccine with specific effectiveness data from Hong Kong. A comprehensive study in Hong Kong compared the effectiveness of the BNT162b2 mRNA vaccine to an inactivated vaccine and oral antiviral drugs, molnupiravir and nirmatrelvir/ritonavir, in a large cohort of hospitalized COVID-19 patients.18 All treatments were associated with lower risks of all-cause mortality and progression to severe conditions, with no significant interaction effects; their benefits were additive.18
In residential care homes for the elderly and disabled, the BNT162b2 mRNA vaccine provided superior protection against severe outcomes during the Omicron wave compared to the inactivated vaccine.19 Residents who received at least two doses of the BNT162b2 mRNA vaccine had effectiveness rates of 52% and 59% against severe outcomes and deaths, respectively, compared to 29% and 36% for the inactivated vaccine.19 Overall, two doses of the BNT162b2 mRNA vaccine offered 79.3% protection against severe disease for individuals aged 60 and older, while the inactivated vaccine provided only 39.5%.20 This highlights the importance of effective vaccination for local populations.20
Inclusive protection: Vaccine effectiveness across diverse populations
Prof. Hung reminded that vulnerable populations, especially children, the elderly and immunocompromised, remain at high risk for severe illness and hospitalization, particularly during the autumn and winter months when infection rates typically surge. Clinical data and real-world data show that COVID-19 vaccines are effective across these diverse populations.21–24
The BNT162b2 mRNA vaccine has been shown to be effective in adolescents and children, with primarily mild to moderate side effects such as injection-site pain (79%-86%), fatigue (60%-66%), and headache (55%-65%), with no serious adverse events linked to the vaccine.21 In a Hong Kong RWE study of 5,493 patients with autoimmune diseases like rheumatoid arthritis, no significant link was found between vaccination and arthritis flare-ups, with adjusted incidence rate ratios of 0.86 for the BNT162b2 mRNA vaccine and 0.87 for the inactivated vaccine, indicating a favorable safety profile.22
For pregnant and lactating women, the BNT162b2 mRNA vaccine has also shown promising results.23,24 Vaccination in lactating women led to substantial levels of COVID-19-specific immunoglobulin A (IgA) and Immunoglobulin G (IgG) antibodies in breast milk 6 weeks post-vaccination, potentially indicating protective benefits for infants against infections and respiratory illnesses.24
Overall, the data support the safety and efficacy of the vaccine across diverse populations, underscoring its crucial role in enhancing public health and protecting communities.
The future of immunization: Coadministration of mRNA vaccines and flu vaccine
Beginning with the 2024/2025 vaccination program, only JN.1-adapted mRNA vaccines will be available under the Government COVID-19 Vaccination Program, following the expiration of inactivated vaccines on October 3, 2024.25 From October 4 onward, mRNA vaccines will be the sole vaccination option.25 High-risk groups, including individuals aged 50 and older, those aged 18 to 49 with underlying health conditions, immunocompromised individuals aged 6 months and older, pregnant women, and healthcare workers, will be eligible for free booster doses at least 6 months after their last dose or COVID-19 infection.25
As the vaccination landscape evolves, it is important to consider the safety and efficacy of administering the JN.1-adapted mRNA vaccine alongside other vaccines, such as the seasonal influenza vaccine (SIV). Coadministration of the BNT162b2 mRNA vaccine with the SIV has been shown to be safe and well-tolerated.26 The safety of co-administration of BNT162b2 mRNA vaccine along with the SIV has been well demonstrated by a phase 3 study involving healthy adults aged 18 to 64, the study has shown that the serious adverse events occurring in less than 1% of participants, and were not vaccine-related.26
Common adverse reactions in the coadministration group included injection site pain (86.2% vs. 84.4% with the BNT162b2 mRNA vaccine alone), fatigue (64.0% vs. 50.8%), and headache (47.2% vs. 37.8%).26 Importantly, the immune responses from co-administered vaccines were robust and noninferior to those from the BNT162b2 mRNA vaccine alone, ensuring effective protection against both COVID-19 and influenza.26 Prof. Hung emphasized that “this approach of coadministration enhances vaccination coverage, allowing individuals to receive multiple vaccines in a single visit. This not only improves convenience for patients but also increases overall vaccination rates, helping to protect communities from multiple infectious diseases simultaneously.”
Conclusion
In conclusion, the emergence of the JN.1 variant underscores the persistent challenges posed by COVID-19 and emphasizes the need for effective vaccines.1 The BNT162b2 mRNA vaccine, particularly the JN.1-adapted formulation, represents an important advancement by demonstrating significant efficacy and safety in clinical trials. Strong real-world evidence of BNT162b2 mRNA vaccine further supports its role in protecting against severe outcomes across diverse populations, reinforcing vaccination as a key strategy. The upcoming transition to mRNA vaccines will ensure continued protection while also facilitating coadministration with influenza vaccines. Ongoing monitoring and research will be essential to deepen our understanding of vaccine effectiveness, which is vital for safeguarding public health in this ever-evolving landscape.