MEETING HIGHLIGHT
CLDN18.2: A unique marker for gastric cancer in the precision oncology era
Diagnostic biomarkers play a crucial role in determining appropriate therapy for gastric or gastroesophageal junction (G/GEJ) cancers.1 However, current biomarkers necessitate a substantial amount of tissue for molecular testing and require the expertise of experienced pathologists to be conducted appropriately. Despite the variety of molecular tests, the intratumoral heterogeneity of G/GEJ cancers often results in inconsistencies in clinical outcomes.2 At a recent symposium, Professor Matteo Fassan discussed the latest hurdles in biomarker testing for G/GEJ cancers, highlighting claudin18.2 (CLDN18.2) as a unique biomarker both as a prognostic device and a target for therapy. He also shared the logistics of assessing CLDN18.2, providing guidance on interpreting immunohistochemistry (IHC) staining. Additionally, he introduced the results from recent phase 3 trials, which demonstrated the efficacy of zolbetuximab, a targeted therapy specifically designed for CLDN18.2-positive G/GEJ cancers.
The inadequacies of current molecular markers for G/GEJ cancers
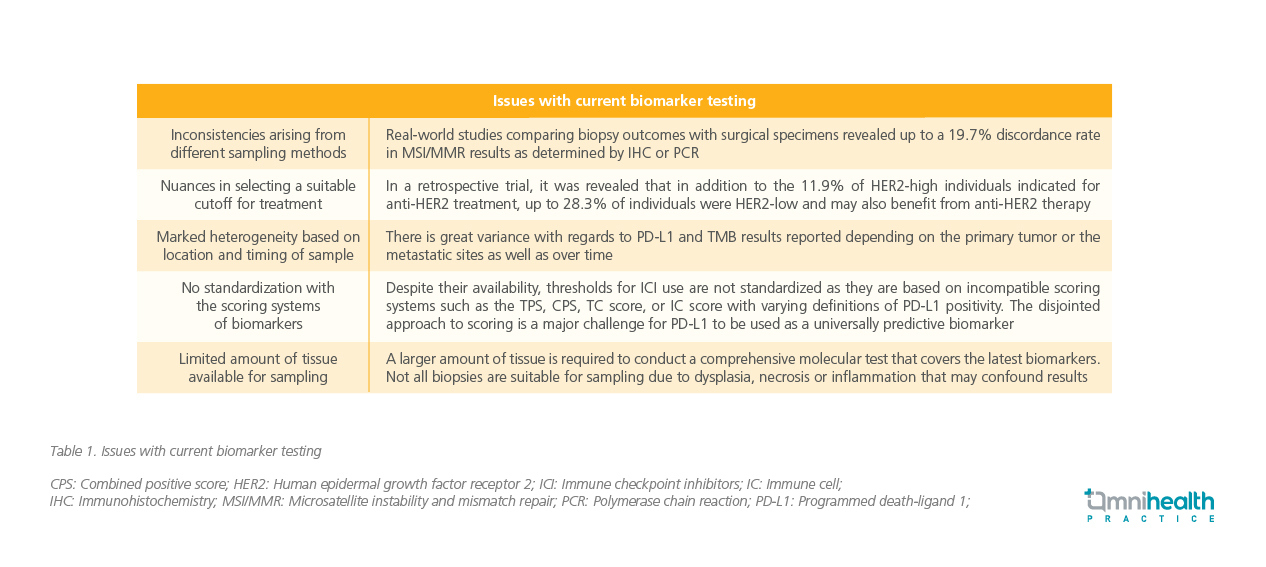
G/GEJ cancers are conventionally classified into distinct molecular subtypes.3 Biomarker-guided therapy is the mainstay of treatment, and the combination of histological and molecular reporting helps inform treatment selection.1,4 Established biomarkers such as programmed death-ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), and microsatellite instability and mismatch repair (MSI/MMR) tests are invaluable in treatment selection according to the American Society of Clinical Oncology (ASCO) treatment guidelines.1,4 However, Prof. Fassan acknowledged that there are many shortcomings with current testing modalities that remain unaddressed as outlined in (table 1).2,6,7
Limited sample availability and the complexity of these biomarker tests require careful handling from experienced gastrointestinal pathologists. However, Prof. Fassan suggested that emerging biomarkers could serve as viable alternatives, challenging the current status quo and addressing some of the issues faced.Ongoing research has identified promising biomarkers, including fibroblast growth factor receptor 2b (FGFR2b), which constitute a significant portion (30.2%) of the HER2-negative metastatic G/GEJ cancer population.5
The clinicopathological value of CLDN18.2 as a biomarker
One of the novel biomarkers for G/GEJ cancers is CLDN18.2. Global phase 3 studies have demonstrated its prevalence of approximately 38.4% across both G/GEJ cancers.8 In addition to primary G/GEJ tumors, CLDN18.2 is also expressed in their metastases.9 This protein belongs to a family of transmembrane proteins that form charge-selective small pores within tight junctions, regulating the flow of molecules through these junctions.10 In normal gastric mucosa, CLDN18.2 remains buried within the tight junction supramolecular complex and is largely inaccessible.11,12 However, during malignant transformation, its epitopes become exposed and serve as targets for monoclonal antibodies.11,12
Studies conducted in the G/GEJ cancer population have revealed that CLDN18.2 can serve as a valuable prognostic marker for characterizing G/GEJ cancer patients. While CLDN18.2 positivity itself was not directly associated with overall survival (OS) outcomes, it showed significant correlations with important clinicopathological factors. These associations included nodal involvement (p=0.0407), stage III or IV disease at diagnosis (p=0.019), younger age of diagnosis (<70 years) (p=0.0035), peritoneal involvement (p<0.001), a lower incidence of liver metastases (p=0.009) and a positive EBV status (p=0.001).13
Moreover, CLDN18.2’s predictive value extends beyond existing biomarkers, providing a unique approach to patient stratification.14 Positive CLDN18.2 samples exhibited nearly equal distribution among various molecular subtypes, without clear correlations to preexisting biomarkers such as HER2, MMR or PD-L1.14 Additionally, CLDN18.2 demonstrated reliability as a biomarker, with an impressive concordance of 83.7% between samples collected from cytology effusion and tissue specimens.15 Similarly, a high concordance of 81.5% was observed at different anatomical sites, including the primary malignancy and the metastasis site.13 Notably, discordant CLDN18.2 status between the primary malignancy and the metastasis occurred in only 1 case, where the tumor was positive and the metastasis was negative.13 These findings underscore the value of CLDN18.2 as a novel biomarker for G/GEJ cancers among the current array of biomarker options.
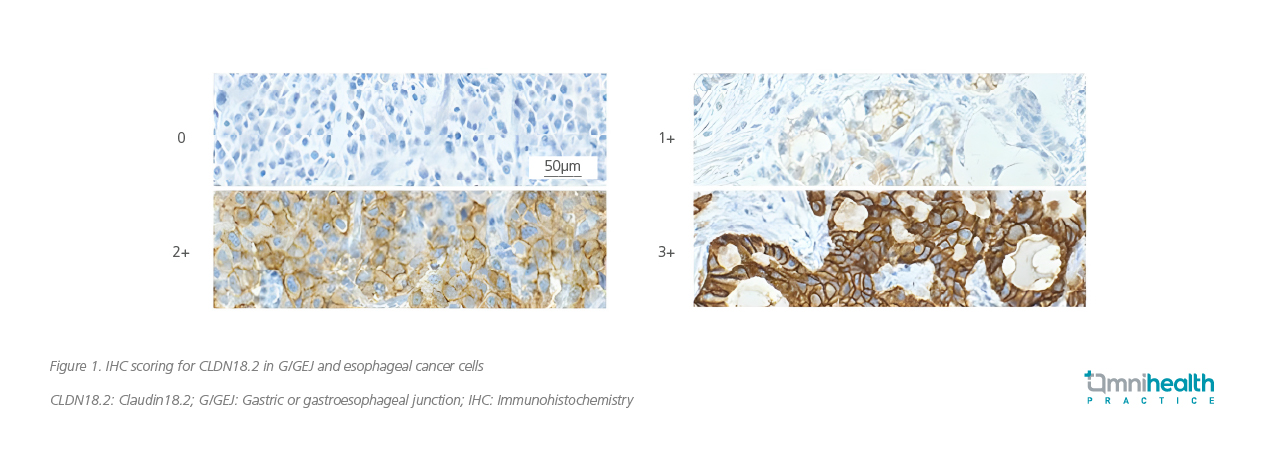
Detection of CLDN18.2 using IHC represents a crucial step in applying molecular tests in clinical practice
To understand how to interpret the results of molecular tests for CLDN18.2 is imperative. Prof. Fassan further illustrated this process by sharing examples of IHC staining slides for the identification of CLDN18.2 expression (figure 1).
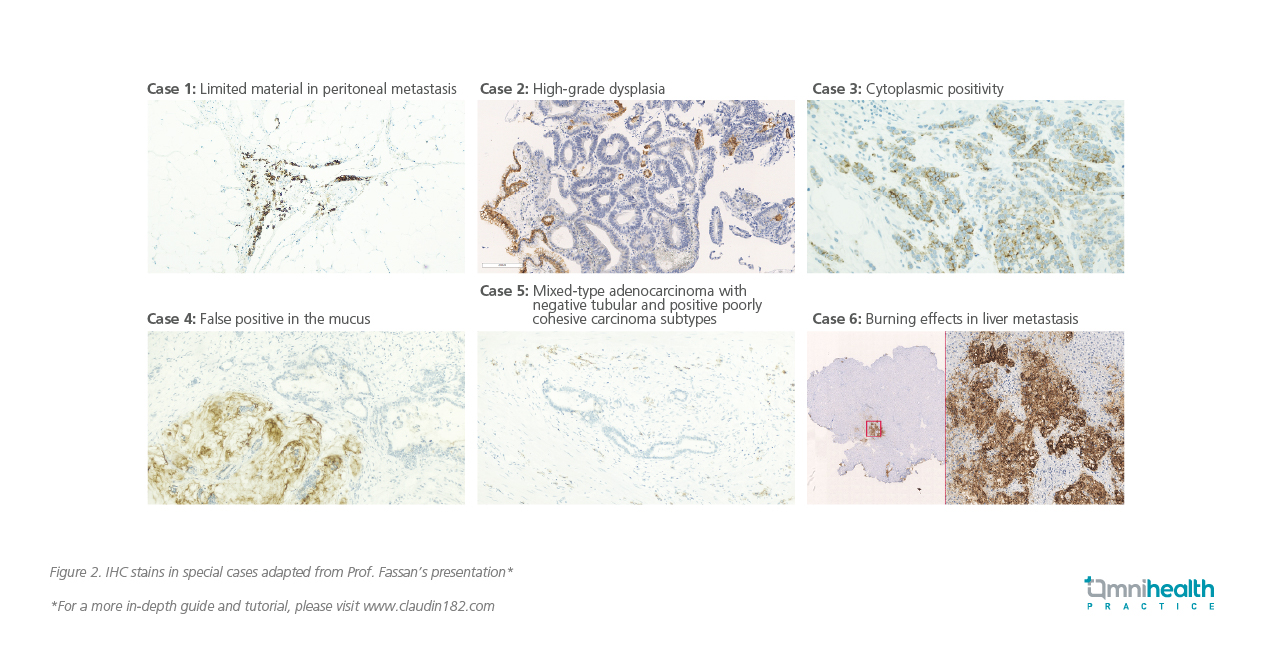
The current definition of CLDN18.2 positivity relies on IHC staining, specifically at a 2+ or 3+ intensity level. In Prof. Fassan’s unpublished research, high expression of CLDN18.2 is defined as positivity in ≥75% of tumor cells.16 However, he also acknowledged that this definition may evolve based on the latest ongoing clinical trials. Prof. Fassan shared additional examples of IHC slides, highlighting some difficult cases (figure 2). He also emphasized the importance of using the 6-point biopsies method as the standard for sampling due to the heterogeneous nature of G/GEJ cancers. This method provides a remarkable 97.7% sensitivity and 98.5% specificity.13 He stressed the need for comprehensive educational programs involving not only pathologists, but also laboratory technicians, oncologists, surgeons, and gastroenterologists.
Zolbetuximab guided by CLDN18.2 expression: A valuable treatment option for G/GEJ cancers
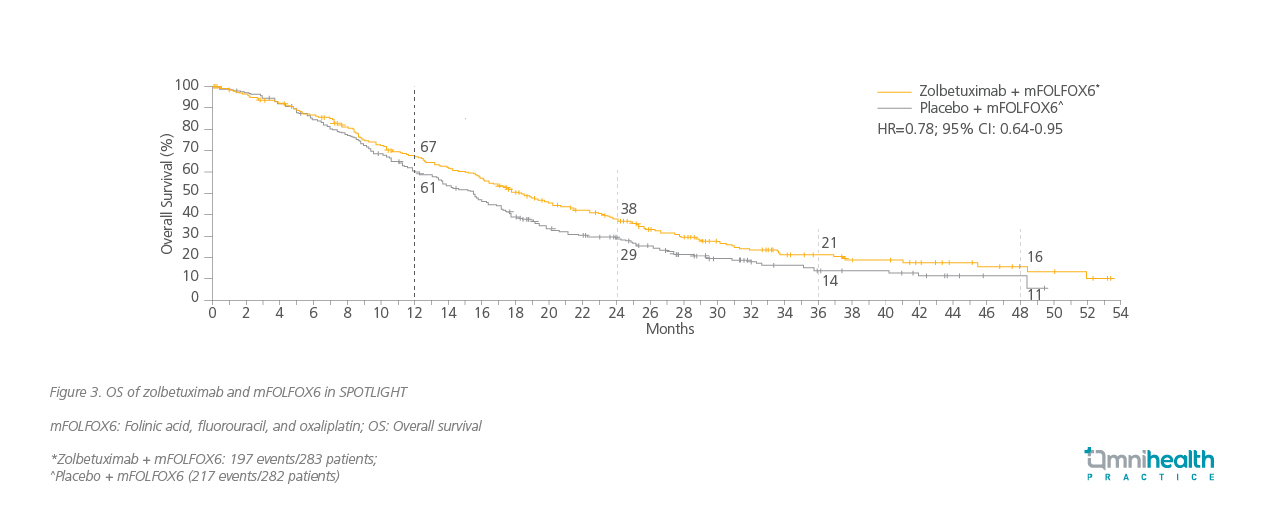
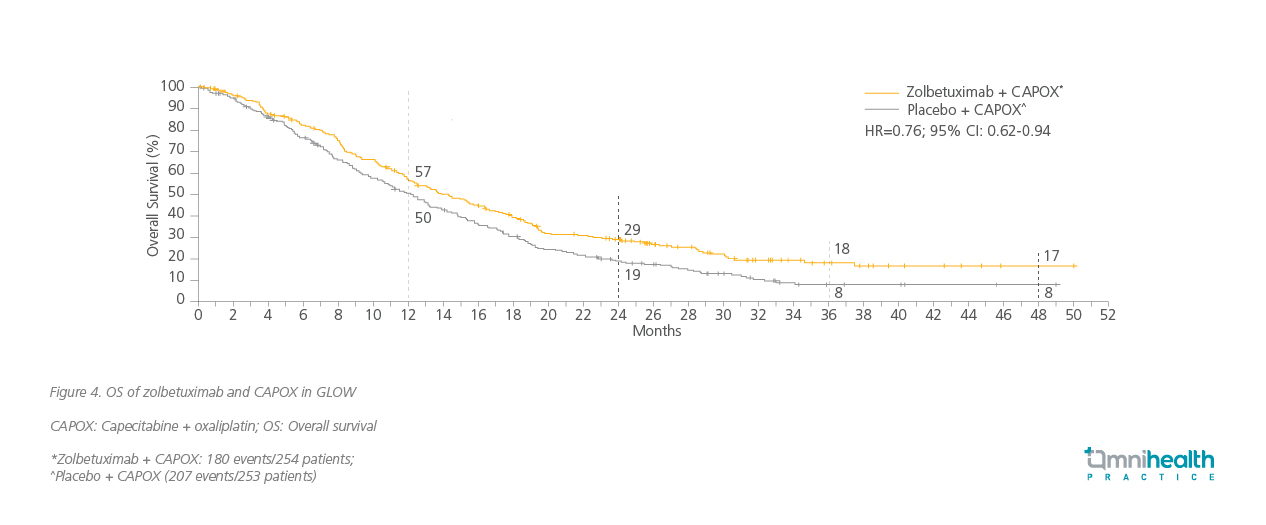
The latest trials have revealed encouraging progression-free survival (PFS) and OS outcomes with targeted therapy, specifically zolbetuximab, when used in combination with chemotherapy.17 Prof. Fassan highlighted the outcomes of 2 trials using different chemotherapy combination regimens. SPOTLIGHT is a phase 3 trial in which patients with locally advanced unresectable or metastatic G/GEJ adenocarcinoma and were CLDN18.2-positive and HER2-negative were given zolbetuximab and mFOLFOX6 as the first-line treatment.17 Similarly, in GLOW, another phase 3 trial, patients with locally advanced unresectable or metastatic G/GEJ adenocarcinoma and were CLDN18.2-positive and HER2-negative were given zolbetuximab and CAPOX as first-line treatment. Major improvements in OS outcomes were achieved in the primary analysis of both trials (figure 3 and 4) along with significant improvements in PFS in SPOTLIGHT (HR=0.73; 95% CI: 0.59-0.91) and GLOW (HR=0.69; 95% CI: 0.55 0.86) respectively.17
Conclusion
Emerging biomarkers offer an ever-expanding perspective on the intricate molecular landscape of G/GEJ cancer. With its distinctive significance for prognosis and as a therapeutic target, novel markers like CLDN18.2 may present a new paradigm in G/GEJ cancer diagnosis and patient stratification. Now, as novel targeted therapies such as zolbetuximab and chemotherapy combinations emerge, it is the optimal time to learn how to interpret IHC for CLDN18.2.